Krista Mayer Energy Unit Student Objectives 2012 Guiding Question
... object by the application of force. In gravitational energy it has to do with the place or position, so a reservoir is an example. Radiant, thermal, motion, sound, and electrical energy are examples of kinetic energy because they all have to do with the motion of waves, electrons, atoms, molecules a ...
... object by the application of force. In gravitational energy it has to do with the place or position, so a reservoir is an example. Radiant, thermal, motion, sound, and electrical energy are examples of kinetic energy because they all have to do with the motion of waves, electrons, atoms, molecules a ...
Work, Power, and Energy [CH 14
... A diver with a mass of 70.0 kg stands motionless at the top of a 3.0-m-high diving platform. Calculate his potential energy relative to the water surface while standing on the platform, and his speed when he enters the pool. (Hint: Assume the diver’s initial vertical speed after diving is zero.) ...
... A diver with a mass of 70.0 kg stands motionless at the top of a 3.0-m-high diving platform. Calculate his potential energy relative to the water surface while standing on the platform, and his speed when he enters the pool. (Hint: Assume the diver’s initial vertical speed after diving is zero.) ...
Lesson - nstacommunities.org
... of a spring to a ring stand and marks the position of its lower end. The students then tie the mass onto the lower end and mark its equilibrium position. The spring constant is obtained by solving the equation mg = kx, where m is the mass of the suspended mass, g is the acceleration due to gravity, ...
... of a spring to a ring stand and marks the position of its lower end. The students then tie the mass onto the lower end and mark its equilibrium position. The spring constant is obtained by solving the equation mg = kx, where m is the mass of the suspended mass, g is the acceleration due to gravity, ...
Energy: Review
... x-rays, gamma rays, radio waves, and solar energy. 2. Thermal (heat) Energy – the internal energy in substances. It is the vibration and movement of atoms and molecules within substances. Example: Geothermal energy. ...
... x-rays, gamma rays, radio waves, and solar energy. 2. Thermal (heat) Energy – the internal energy in substances. It is the vibration and movement of atoms and molecules within substances. Example: Geothermal energy. ...
Name: Core: ______ Date: ENERGY REVIEW – INNOVATION LAB
... 15. Why is electricity an incredibly useful form of energy? Electrical energy is an incredibly useful form of energy because it can be generated from many other types of energy and easily transported to new locations. 16. What is the most common form of obtaining energy? The most common method for ...
... 15. Why is electricity an incredibly useful form of energy? Electrical energy is an incredibly useful form of energy because it can be generated from many other types of energy and easily transported to new locations. 16. What is the most common form of obtaining energy? The most common method for ...
Energy and Heat
... Energy • Conduction is the transfer of energy through matter by direct contact of particles. • Conduction takes place in solids, liquids, and gases. • The most important way thermal energy is transferred in fluids is by convection. • Convection is the transfer of energy by the bulk movement of matte ...
... Energy • Conduction is the transfer of energy through matter by direct contact of particles. • Conduction takes place in solids, liquids, and gases. • The most important way thermal energy is transferred in fluids is by convection. • Convection is the transfer of energy by the bulk movement of matte ...
Energy - Buckeye Valley
... Results from an elastic material being stretched or compressed some distance. Depends on how far the elastic material is stretched / compressed and the material’s spring constant. ...
... Results from an elastic material being stretched or compressed some distance. Depends on how far the elastic material is stretched / compressed and the material’s spring constant. ...
Name: Chapter 14: Changing Forms of Energy Words to Know
... Potential energy (or ‘stored’ energy) is energy that is not causing any changes NOW, but could cause changes in the FUTURE There are several different kinds of potential energy The object’s position determines its type of potential energy Potential energy often turns into kinetic energy- when the ob ...
... Potential energy (or ‘stored’ energy) is energy that is not causing any changes NOW, but could cause changes in the FUTURE There are several different kinds of potential energy The object’s position determines its type of potential energy Potential energy often turns into kinetic energy- when the ob ...
Temperature, Thermal Energy and Heat
... faster moving particles collide with slower moving particles. During conduction, heat is transferred from matter with a higher temperature and greater kinetic energy to matter with a lower temperature and less kinetic energy. For example, if a metal spoon that is at room temperature is placed in a p ...
... faster moving particles collide with slower moving particles. During conduction, heat is transferred from matter with a higher temperature and greater kinetic energy to matter with a lower temperature and less kinetic energy. For example, if a metal spoon that is at room temperature is placed in a p ...
Potential and Kinetic Energy
... Investigate and describe the transformation of energy that occurs in given examples. 2 – All of 1 + Differentiate between kinetic and potential energy. 1 - Identify examples of kinetic and potential energy. ...
... Investigate and describe the transformation of energy that occurs in given examples. 2 – All of 1 + Differentiate between kinetic and potential energy. 1 - Identify examples of kinetic and potential energy. ...
Energy all types
... Thermal energy is the total of all the kinetic and potential energy of the atoms in an object. When any form of matter gets warmer, the kinetic energy of its atoms increases. The object’s particles move faster, so its thermal energy increases. A change in thermal energy can lead to a change in phase ...
... Thermal energy is the total of all the kinetic and potential energy of the atoms in an object. When any form of matter gets warmer, the kinetic energy of its atoms increases. The object’s particles move faster, so its thermal energy increases. A change in thermal energy can lead to a change in phase ...
Energy Conversions When energy is changed from one form to
... Food is a good example of chemical energy conversions. Nuclear energy in the sun is created when matter is converted into energy. The nuclear energy makes thermal energy (the sun is hot), the hot sun gives off light energy, the light energy makes it to the Earth where plants use photosynthesis to co ...
... Food is a good example of chemical energy conversions. Nuclear energy in the sun is created when matter is converted into energy. The nuclear energy makes thermal energy (the sun is hot), the hot sun gives off light energy, the light energy makes it to the Earth where plants use photosynthesis to co ...
Chap-13 Simple Machines and its - Environmental-Chemistry
... single large nucleus is split into two or more smaller nuclei. • In both nuclear fusion and fission, small quantity of mass are converted into large quantities of energy. ...
... single large nucleus is split into two or more smaller nuclei. • In both nuclear fusion and fission, small quantity of mass are converted into large quantities of energy. ...
energy changes
... When we eat, our bodies transform the energy stored in the food into energy to do work. When we run or walk, we "burn" food energy in our bodies. When we think or read or write, we are also doing work. Many times it's really hard work! We EAT food that our bodies turn into stored energy to use when ...
... When we eat, our bodies transform the energy stored in the food into energy to do work. When we run or walk, we "burn" food energy in our bodies. When we think or read or write, we are also doing work. Many times it's really hard work! We EAT food that our bodies turn into stored energy to use when ...
As the great debate on energy conservation continues in the political
... Energy comes in different forms like heat (thermal), light (radiant), and motion (Kinetic), electrical, chemical, nuclear energy and gravitational. There is two types of energy as well as different sources of energy. Two types of energy, one being stored which is potential energy and working also k ...
... Energy comes in different forms like heat (thermal), light (radiant), and motion (Kinetic), electrical, chemical, nuclear energy and gravitational. There is two types of energy as well as different sources of energy. Two types of energy, one being stored which is potential energy and working also k ...
ENERGY There is a law governing all natural phenomena. There is
... possibility of doing some work. Elastic energy is the formula for a spring when it is stretched. How much energy is it? If we let go, the elastic energy, as the spring passes through the equilibrium point, is converted to kinetic energy and it goes back and forth between com pressing or stretching t ...
... possibility of doing some work. Elastic energy is the formula for a spring when it is stretched. How much energy is it? If we let go, the elastic energy, as the spring passes through the equilibrium point, is converted to kinetic energy and it goes back and forth between com pressing or stretching t ...
Energy - Solon City Schools
... • When you boil a pot of water, you are contributing thermal energy or heat to the bottom of the pot. This thermal energy is then transferred to the water inside the pot. • As the water molecules move faster (kinetic energy), they begin to get hotter. As they move faster and faster, each one tries t ...
... • When you boil a pot of water, you are contributing thermal energy or heat to the bottom of the pot. This thermal energy is then transferred to the water inside the pot. • As the water molecules move faster (kinetic energy), they begin to get hotter. As they move faster and faster, each one tries t ...
Kinetic Energy
... This is the energy possessed by a moving object. Kinetic energy increases is the object’s speed is increased. ...
... This is the energy possessed by a moving object. Kinetic energy increases is the object’s speed is increased. ...
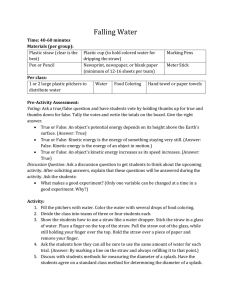
Falling Water
... kinetic energy of water changes when it hits the floor. Engineers must understand how height affects the amount of kinetic energy so they create a reservoir that holds the proper amount of water and design a hydroelectric power plant that produces the right amount of electricity. Since water falls t ...
... kinetic energy of water changes when it hits the floor. Engineers must understand how height affects the amount of kinetic energy so they create a reservoir that holds the proper amount of water and design a hydroelectric power plant that produces the right amount of electricity. Since water falls t ...
LIFEPAC® 12th Grade Science Unit 3 Worktext - HomeSchool
... Identify and classify phases of matter. Distinguish between temperature and heat. Calculate heat energy involving latent heats. ...
... Identify and classify phases of matter. Distinguish between temperature and heat. Calculate heat energy involving latent heats. ...
energy - wellswaysciences
... Conservation of Energy • Lesson Objectives: • All must know that energy an be neither created nor destroyed but it can be changed from one form into another. • All must know that some energy is wasted (usually as heat) when energy is transferred. • Most should be able to draw, label and use simple ...
... Conservation of Energy • Lesson Objectives: • All must know that energy an be neither created nor destroyed but it can be changed from one form into another. • All must know that some energy is wasted (usually as heat) when energy is transferred. • Most should be able to draw, label and use simple ...
Energy
... energy due to friction (heat) in the ground and air, vibrations in the earth (energy waves.) •If the object bounces, some energy is converted momentarily into elastic potential energy. ...
... energy due to friction (heat) in the ground and air, vibrations in the earth (energy waves.) •If the object bounces, some energy is converted momentarily into elastic potential energy. ...
Chapter 7: Energy
... • Except for nuclear, and geothermal power, source of our energy is ultimately the sun: eg. Gas, wood, coal, petroleum combustion – all these come from plants, which used sun’s radiant energy in photosynthesis. Also, sun is responsible for energy in photovoltaic cells in solar-powered panels, and in ...
... • Except for nuclear, and geothermal power, source of our energy is ultimately the sun: eg. Gas, wood, coal, petroleum combustion – all these come from plants, which used sun’s radiant energy in photosynthesis. Also, sun is responsible for energy in photovoltaic cells in solar-powered panels, and in ...
Walking - Physics Forums
... The law of conservation of energy states that energy cannot be created or destroyed. It is transformed from one form into another, but the total amount of energy does not change. Energy in motion is defined as either kinetic; energy able to provide work, or potential; stored energy held in readiness ...
... The law of conservation of energy states that energy cannot be created or destroyed. It is transformed from one form into another, but the total amount of energy does not change. Energy in motion is defined as either kinetic; energy able to provide work, or potential; stored energy held in readiness ...
World energy consumption
World energy consumption refers to the total energy used by all of human civilization. Typically measured per year, it involves all energy harnessed from every energy source applied towards humanity's endeavors across every single industrial and technological sector, across every country. Being the power source metric of civilization, World Energy Consumption has deep implications for humanity's social-economic-political sphere.Institutions such as the International Energy Agency (IEA), the U.S. Energy Information Administration (EIA), and the European Environment Agency record and publish energy data periodically. Improved data and understanding of World Energy Consumption may reveal systemic trends and patterns, which could help frame current energy issues and encourage movement towards collectively useful solutions.In 2012, the IEA estimated that the world energy consumption was 155,505 terawatt-hour (TWh), or 5.598 × 1020 joules. This works out to 17.7 TW, or a bit less than the estimated 20 TW produced by radioactive decay on earth. From 2000–2012 coal was the source of energy with the largest growth. The use of oil and natural gas also had considerable growth, followed by hydro power and renewable energy. Renewable energy grew at a rate faster than any other time in history during this period, which can possibly be explained by an increase in international investment in renewable energy. The demand for nuclear energy decreased, possibly due to the accidents at Chernobyl and Three Mile Island.In 2011, expenditures on energy totaled over 6 trillion USD, or about 10% of the world gross domestic product (GDP). Europe spends close to one quarter of the world energy expenditures, Americans close to 20%, and Japan 6%.