advanced placement chemistry alamo heights high school scope
... Activity: Effect on biological systems [CR4] Students examine a demonstration size model of DNA or an alpha helix, and use their fingers to identify which atoms / base pairs are particularly involved in ...
... Activity: Effect on biological systems [CR4] Students examine a demonstration size model of DNA or an alpha helix, and use their fingers to identify which atoms / base pairs are particularly involved in ...
Lecture 13 11-20-02
... For weak acids or bases we use the limits of their buffer region and the pKa to estimate the three points. Straight lines are drawn through each set of points, with each line intersecting the vertical line representing the equivalence point volume. Finally, a smooth curve is drawn connecting the thr ...
... For weak acids or bases we use the limits of their buffer region and the pKa to estimate the three points. Straight lines are drawn through each set of points, with each line intersecting the vertical line representing the equivalence point volume. Finally, a smooth curve is drawn connecting the thr ...
Solutions. Electrolytic dissociation
... Acids and bases √ According to the Brønsted–Lowry theory of acids and bases, an acid is a proton donor and a base is a proton acceptor. The proton in this context means a solvated hydrogen ion (H+) that presents in solution. Acids and bases in solution are always in equilibrium with their deprotona ...
... Acids and bases √ According to the Brønsted–Lowry theory of acids and bases, an acid is a proton donor and a base is a proton acceptor. The proton in this context means a solvated hydrogen ion (H+) that presents in solution. Acids and bases in solution are always in equilibrium with their deprotona ...
Amino acids - CMA
... weak base and can deprotonate the carboxylic acid. The H+ (proton) shifts from the COOH group to the NH2 group, resulting in a negatively charged group (COO -) and a positively charged group (NH3+). This creates a molecular ion with a distinct positive and negative charge, though the molecule as a w ...
... weak base and can deprotonate the carboxylic acid. The H+ (proton) shifts from the COOH group to the NH2 group, resulting in a negatively charged group (COO -) and a positively charged group (NH3+). This creates a molecular ion with a distinct positive and negative charge, though the molecule as a w ...
Learning Guide – Poisons (I)
... Plants make sugar and oxygen from carbon dioxide and water. “Hot hands” get warm when bent. Old wine turns into vinegar. Paint remover loosens paint so it can be removed. Balancing chemical reactions When we write a chemical reaction, it is important to know how many units of each compound are neede ...
... Plants make sugar and oxygen from carbon dioxide and water. “Hot hands” get warm when bent. Old wine turns into vinegar. Paint remover loosens paint so it can be removed. Balancing chemical reactions When we write a chemical reaction, it is important to know how many units of each compound are neede ...
Anomalous thermodynamic properties in ferropericlase throughout
... transition5 but more recent investigations9,10,13 have confirmed its continuous nature. Here we predict the thermodynamics properties of Fp at relevant mantle conditions by combining first-principles calculations, the ideal solid solution 共ISS兲 formalism, and quasiharmonic theory. We build on a prev ...
... transition5 but more recent investigations9,10,13 have confirmed its continuous nature. Here we predict the thermodynamics properties of Fp at relevant mantle conditions by combining first-principles calculations, the ideal solid solution 共ISS兲 formalism, and quasiharmonic theory. We build on a prev ...
calculations of mechanical work in 1, 2 and 3 dimensions
... Ask yourself: Is F constant with changing L? (What does the equationof-state say?) ...
... Ask yourself: Is F constant with changing L? (What does the equationof-state say?) ...
A comparison of methods to determination of macromolecule
... methods result in rather similar values of gel dose and scission-to-crosslinking ratio. At the same time, both Ch-R and LLS methods seem to be more accurate due to the higher values of the coefficients of linear correlation. Key words: crosslinking by irradiation, sol-gel analysis, crosslinking comp ...
... methods result in rather similar values of gel dose and scission-to-crosslinking ratio. At the same time, both Ch-R and LLS methods seem to be more accurate due to the higher values of the coefficients of linear correlation. Key words: crosslinking by irradiation, sol-gel analysis, crosslinking comp ...
2/22 Lecture Slides
... Thermodynamics ΔG = Change in Gibbs free energy This tells us if a process is spontaneous (expected to happen) or non-spontaneous ΔG < 0 process is spontaneous (favored) ΔG = ΔH - TΔS (T is absolute temperature) processes that are exothermic (Δ H < 0) and increase disorder (Δ S > 0) are favored at ...
... Thermodynamics ΔG = Change in Gibbs free energy This tells us if a process is spontaneous (expected to happen) or non-spontaneous ΔG < 0 process is spontaneous (favored) ΔG = ΔH - TΔS (T is absolute temperature) processes that are exothermic (Δ H < 0) and increase disorder (Δ S > 0) are favored at ...
CHEM%1212K% Final%Exam% Summer%2011% K
... 17.%%Identify%the%most%likely%analyte%and%titrant%(listed%in%that%order)%based%on%the% ...
... 17.%%Identify%the%most%likely%analyte%and%titrant%(listed%in%that%order)%based%on%the% ...
Critical Micelar Concentration and Thermodynamic Parameters of
... The values of enthalpy variation before the CMC (curve inflection) represent the sum of molar enthalpies of micelles dilution, micelles rupture, monomers solvation, Before the CMC just the micelles dilution is registered. The variation of molar enthalpy of demicellization ( ∆ desmic H = −∆ mic H ) i ...
... The values of enthalpy variation before the CMC (curve inflection) represent the sum of molar enthalpies of micelles dilution, micelles rupture, monomers solvation, Before the CMC just the micelles dilution is registered. The variation of molar enthalpy of demicellization ( ∆ desmic H = −∆ mic H ) i ...
8 - THE DETERMINATION OF THE CONCENTRATION
... if the pH has not reached 11.5 units. Why does adding water to the solution not affect the determination of the concentration or pKa-values? Repeat the titration twice or until the average deviation of the volumes required reaching the first equivalence point is less than 0.10 mL. Neutralize all tit ...
... if the pH has not reached 11.5 units. Why does adding water to the solution not affect the determination of the concentration or pKa-values? Repeat the titration twice or until the average deviation of the volumes required reaching the first equivalence point is less than 0.10 mL. Neutralize all tit ...
wiley_ch6_Chemical_Equilibrium
... System at equilibrium (Q = K) when upset by disturbance (Q ≠ K) will shift to offset stress System said to “shift to right” when forward reaction is dominant (Q < K) System said to “shift to left” when reverse direction is dominant (Q > K) Jespersen/Brady/Hyslop ...
... System at equilibrium (Q = K) when upset by disturbance (Q ≠ K) will shift to offset stress System said to “shift to right” when forward reaction is dominant (Q < K) System said to “shift to left” when reverse direction is dominant (Q > K) Jespersen/Brady/Hyslop ...
Study Guide Chapter 16: The Process of Chemical Reactions
... a. The concentration of H2O is increased by the addition of more H2O. (1) Using Le Châtelier's Principle, we predict that the system will shift to more products to partially counteract the increase in H2O. (2) The increase in the concentration of water vapor speeds the forward reaction without i ...
... a. The concentration of H2O is increased by the addition of more H2O. (1) Using Le Châtelier's Principle, we predict that the system will shift to more products to partially counteract the increase in H2O. (2) The increase in the concentration of water vapor speeds the forward reaction without i ...
Acids and Bases
... Dissociation • In water all ionic compounds dissociate into its ionic parts • So NaCl in water dissociates into Na+ and Cl• So H3PO4 dissociates into 3H+ and PO4-3 • Remembers ionic compounds are formed by metals and nonmetals or by metals and polyatomic ions ...
... Dissociation • In water all ionic compounds dissociate into its ionic parts • So NaCl in water dissociates into Na+ and Cl• So H3PO4 dissociates into 3H+ and PO4-3 • Remembers ionic compounds are formed by metals and nonmetals or by metals and polyatomic ions ...
Counting atoms
... measured via X-ray interferometry. The current most accurately measured value of NA, 6.02214076 × 1023 mol–1 with a relative standard uncertainty of 20 parts in 109, has been obtained in this way by the International Avogadro Coordination project 5. High-precision measurements of the Avogadro consta ...
... measured via X-ray interferometry. The current most accurately measured value of NA, 6.02214076 × 1023 mol–1 with a relative standard uncertainty of 20 parts in 109, has been obtained in this way by the International Avogadro Coordination project 5. High-precision measurements of the Avogadro consta ...
Chemistry-Maths-Student-Guide
... Congratulations on choosing A level chemistry! Quite a few chemistry students are also very competent at maths but, if that’s not you, don’t worry! There’s very little mathematics that you’ll encounter in A level chemistry that you haven’t yet seen in your Mathematics GCSE. Of course, you do need to ...
... Congratulations on choosing A level chemistry! Quite a few chemistry students are also very competent at maths but, if that’s not you, don’t worry! There’s very little mathematics that you’ll encounter in A level chemistry that you haven’t yet seen in your Mathematics GCSE. Of course, you do need to ...
Mechanistic Studies of the Reactions of Silicon
... stabilizing substituents at the silenic carbon) should show linear alcohol-quenching kinetics at relatively low alcohol concentrations, with the effects of the two competing proton-transfer pathways only becoming observable at relatively high alcohol concentrations. These conditions are amenable to ...
... stabilizing substituents at the silenic carbon) should show linear alcohol-quenching kinetics at relatively low alcohol concentrations, with the effects of the two competing proton-transfer pathways only becoming observable at relatively high alcohol concentrations. These conditions are amenable to ...
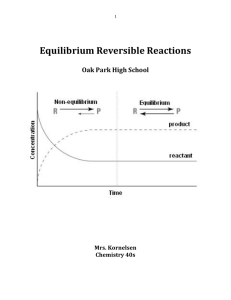
Equilibrium Reversible Reactions
... • Keq relates the concentrations of products to reactants at equilibrium. • The concentrations of both aqueous solutions and gases change during the progress of a reaction. For reactions involving a solid or a liquid, while the amounts of the solid or liquid will change during a reaction, their conc ...
... • Keq relates the concentrations of products to reactants at equilibrium. • The concentrations of both aqueous solutions and gases change during the progress of a reaction. For reactions involving a solid or a liquid, while the amounts of the solid or liquid will change during a reaction, their conc ...