Kinetic Study of the Reaction of Diborane with Phosphine*
... since the half-time of the reaction was generally of the order of amounts of solid surface present. several hours. Blank experiments using one reactant at a time The conclusion that the reaction rate is independent showed that the time required to establish thermal equilibrium was only about 0.5 min ...
... since the half-time of the reaction was generally of the order of amounts of solid surface present. several hours. Blank experiments using one reactant at a time The conclusion that the reaction rate is independent showed that the time required to establish thermal equilibrium was only about 0.5 min ...
Chapter 15: Chemical Equilibrium
... The equilibrium concentrations of ammonia and hydrogen chloride do not depend on the amount of solid present as long as there is some solid present for the system to reach equilibrium. The concentration of a pure solid depends only on the density of the substance, a constant that can be incorporate ...
... The equilibrium concentrations of ammonia and hydrogen chloride do not depend on the amount of solid present as long as there is some solid present for the system to reach equilibrium. The concentration of a pure solid depends only on the density of the substance, a constant that can be incorporate ...
MIDDLE COLLEGE HIGH SCHOOL
... Base your answers to questions 79 and 80 on the information and equation below. Human blood contains dissolved carbonic acid, H2CO3, in equilibrium with carbon dioxide and water. The equilibrium system is shown below. H2CO3(aq) CO2(aq) + H2O(ℓ) 61. Explain, using LeChatelier’s principle, why decreas ...
... Base your answers to questions 79 and 80 on the information and equation below. Human blood contains dissolved carbonic acid, H2CO3, in equilibrium with carbon dioxide and water. The equilibrium system is shown below. H2CO3(aq) CO2(aq) + H2O(ℓ) 61. Explain, using LeChatelier’s principle, why decreas ...
Contents and Concepts
... for the spontaneity of a reaction at constant temperature, T, and pressure, P. By defining a quantity called the free energy, G = H – TS, we find that ∆G equals the quantity ∆H – T∆S, so the free energy gives us a thermodynamic criterion of ...
... for the spontaneity of a reaction at constant temperature, T, and pressure, P. By defining a quantity called the free energy, G = H – TS, we find that ∆G equals the quantity ∆H – T∆S, so the free energy gives us a thermodynamic criterion of ...
Thermodynamic Investigation of the AINC and AICN Isomers by
... The difference of 50 kJ mol21 with our value suggests that molecular carbides such as Al2C2, AlC2 are formed by interaction of aluminum with graphite at 2700 K in addition to AlNC~AlCN!. Our proportioning of the m/e553 primary ion current to I~AlNC1! and I~AlCN1! has been based on the energy differe ...
... The difference of 50 kJ mol21 with our value suggests that molecular carbides such as Al2C2, AlC2 are formed by interaction of aluminum with graphite at 2700 K in addition to AlNC~AlCN!. Our proportioning of the m/e553 primary ion current to I~AlNC1! and I~AlCN1! has been based on the energy differe ...
Title Variable anisotropy of ionic conduction in lithium nitride: Effect
... in Table I. In the undoped case, the formation energies were obtained at the Li-rich limit and T = 300 K, using Eq. 共1兲 and the Fermi level determined via the charge neutrality condition given by Eq. 共6兲. It is noted, however, that the values are almost independent of the Li and N chemical potential ...
... in Table I. In the undoped case, the formation energies were obtained at the Li-rich limit and T = 300 K, using Eq. 共1兲 and the Fermi level determined via the charge neutrality condition given by Eq. 共6兲. It is noted, however, that the values are almost independent of the Li and N chemical potential ...
Kopplungsmechanismen zwischen Stratosphäre und
... were chosen a priori. From given data (O3) and predictors the coefficients j, f, a, q1, etc., and thus the different contributions, can be determined by stepwise linear regression. Only statistically significant (P > 90%) predictors were left in the regression. The main features of the time series, ...
... were chosen a priori. From given data (O3) and predictors the coefficients j, f, a, q1, etc., and thus the different contributions, can be determined by stepwise linear regression. Only statistically significant (P > 90%) predictors were left in the regression. The main features of the time series, ...
Potassium trans
... under nitrogen atmosphere to avoid CO2 contamination and the temperature was kept at 25±0.1°C. A constant ionic strength of 0.1 M was maintained with NaClO4. The complex was titrated with 0.0502 M aqueous NaOH solution. A combination pH electrode was bought from Mettler Toledo and calibrated using p ...
... under nitrogen atmosphere to avoid CO2 contamination and the temperature was kept at 25±0.1°C. A constant ionic strength of 0.1 M was maintained with NaClO4. The complex was titrated with 0.0502 M aqueous NaOH solution. A combination pH electrode was bought from Mettler Toledo and calibrated using p ...
File
... specific amount of cookies (product), say for example two dozen. If the ratios are wrong, you have waste remaining. If you do nothing about the uneven balance of ingredients caused by the waste, the cookies don't taste right, or may fall apart. In chemistry, the same is true. In order to make a chem ...
... specific amount of cookies (product), say for example two dozen. If the ratios are wrong, you have waste remaining. If you do nothing about the uneven balance of ingredients caused by the waste, the cookies don't taste right, or may fall apart. In chemistry, the same is true. In order to make a chem ...
CONDUCTOMETRY
... Equivalent conductance (o ): “It is the conductance of a solution of one-gram equivalent of solute (with no respect of its volume) contained between two electrodes placed 1 cm apart.” Due to the interionic effects, the equivalent conductance (o ) is concentration dependant. The value of (o) (equ ...
... Equivalent conductance (o ): “It is the conductance of a solution of one-gram equivalent of solute (with no respect of its volume) contained between two electrodes placed 1 cm apart.” Due to the interionic effects, the equivalent conductance (o ) is concentration dependant. The value of (o) (equ ...
The origin and status of the Arrhenius equation
... simple Arrhenius equation was satisfactory for almost all known sets of data on individual processes in the gas phase, if due allowance is made for experimental error" (19). This situation quickly changed as the newer techniques for the study of fast reactions vastly extended the temperature range o ...
... simple Arrhenius equation was satisfactory for almost all known sets of data on individual processes in the gas phase, if due allowance is made for experimental error" (19). This situation quickly changed as the newer techniques for the study of fast reactions vastly extended the temperature range o ...
Ch. 3 Sections 3.9-3.10 Notes
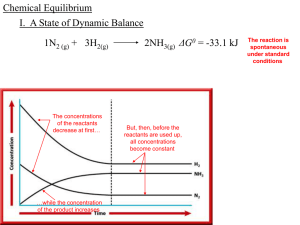
... Suppose a chemist mixed 1.00 mol of N2 with 5.00 mol of H2. What is the maximum number of moles of product that could form? Note the coefficients tell us that 1 mol of N2 consumes 3 mol of H2. 1 mol N2 ↔ 3 mol H2 But 5 mol of H2 was used, not 3, so there will be 2 mol of H2 left over. Once the 1 mol ...
... Suppose a chemist mixed 1.00 mol of N2 with 5.00 mol of H2. What is the maximum number of moles of product that could form? Note the coefficients tell us that 1 mol of N2 consumes 3 mol of H2. 1 mol N2 ↔ 3 mol H2 But 5 mol of H2 was used, not 3, so there will be 2 mol of H2 left over. Once the 1 mol ...
Reaction Systems Engineering II (part 1)
... Solution to Exercise 1.2 E° = –rG / F = 228.51000/(296485) = 1.18 V (300 K), = 192.61000/(296485) = 1.00 V (1000 K) * Theoretical emf depends on the overall cell reaction only. * The E° = 1.23 V derived from the room temperature rG° = –237.1 for H2(g) + 0.5 O2(g) H2O(l) is usually called a ...
... Solution to Exercise 1.2 E° = –rG / F = 228.51000/(296485) = 1.18 V (300 K), = 192.61000/(296485) = 1.00 V (1000 K) * Theoretical emf depends on the overall cell reaction only. * The E° = 1.23 V derived from the room temperature rG° = –237.1 for H2(g) + 0.5 O2(g) H2O(l) is usually called a ...
1.0 basic concepts
... • This means that water, H2O cannot be formed, therefore H2 is the product • If you look at the reactants in (a) – (d), you’ll notice that the metal has oxygen present. • This means that water, H2O can be formed, therefore H2O is the product Carbon dioxide or not • If you look at the reactants in (d ...
... • This means that water, H2O cannot be formed, therefore H2 is the product • If you look at the reactants in (a) – (d), you’ll notice that the metal has oxygen present. • This means that water, H2O can be formed, therefore H2O is the product Carbon dioxide or not • If you look at the reactants in (d ...