
2001 AP Chemistry Scoring Guidelines - AP Central
... These materials were produced by Educational Testing Service (ETS), which develops and administers the examinations of the Advanced Placement Program for the College Board. The College Board and Educational Testing Service (ETS) are dedicated to the principle of equal opportunity, and their programs ...
... These materials were produced by Educational Testing Service (ETS), which develops and administers the examinations of the Advanced Placement Program for the College Board. The College Board and Educational Testing Service (ETS) are dedicated to the principle of equal opportunity, and their programs ...
Spectroscopy of Non-Heme Iron Thiolate Complexes: Insight into the
... energy difference between the excited state and ground state (∆SCF procedure). Note that several excited configurations could not be appropriately converged and have thus not been reported. All calculations were performed on either an SGI Origin 2000 8-CPU R10k server running IRIX 6.5.3 or an Intel ...
... energy difference between the excited state and ground state (∆SCF procedure). Note that several excited configurations could not be appropriately converged and have thus not been reported. All calculations were performed on either an SGI Origin 2000 8-CPU R10k server running IRIX 6.5.3 or an Intel ...
Mole calculations File
... • Radius carbon atom = 73 pm; density (diamond) = 3.5 gcm-3 • Calculate number of carbon atoms in 12 g • volume 12 g = mass/density … unit …convert to m3 • volume occupied by an atom …assumption cubic or spherical? • atoms in 12 g = vol 12g/vol atom • Approx 1x1024…should be 6.02x1023 mol-1 ...
... • Radius carbon atom = 73 pm; density (diamond) = 3.5 gcm-3 • Calculate number of carbon atoms in 12 g • volume 12 g = mass/density … unit …convert to m3 • volume occupied by an atom …assumption cubic or spherical? • atoms in 12 g = vol 12g/vol atom • Approx 1x1024…should be 6.02x1023 mol-1 ...
Module Seven - DePauw University
... are another matter entirely. A solution consists of one or more compounds, which are called solutes, dissolved in a liquid, which is also known as the solvent. The amount of solute in a given amount of solvent is the solute’s concentration. Because a solution contains a minimum of two components (th ...
... are another matter entirely. A solution consists of one or more compounds, which are called solutes, dissolved in a liquid, which is also known as the solvent. The amount of solute in a given amount of solvent is the solute’s concentration. Because a solution contains a minimum of two components (th ...
Boronic acids facilitate rapid oxime condensations at neutral pH
... condensation is the size of required 2-FBPA motif. Although for most applications this should present no difficulties, examples where the compactness of the oxime is critical (such as, for example, as a functional isostere of peptide bonds)37 would not be possible. The ability to run conjugations at 1 ...
... condensation is the size of required 2-FBPA motif. Although for most applications this should present no difficulties, examples where the compactness of the oxime is critical (such as, for example, as a functional isostere of peptide bonds)37 would not be possible. The ability to run conjugations at 1 ...
General Chemistry - Bioinorganic and Solution Chemistry Group
... Determination of melting and boiling points ........................................................................................ 42 Preparation of volatile substances .................................................................................................... 44 Determination of the mola ...
... Determination of melting and boiling points ........................................................................................ 42 Preparation of volatile substances .................................................................................................... 44 Determination of the mola ...
Redox speciation analysis of antimony in soil extracts by hydride
... method is based on the selective generation of stibine from Sb(III) in a continuous flow system using atomic fluorescence spectrometry for detection. Sb(V) is masked by citric or oxalic acid in HCl medium. The procedure was optimized with synthetic solutions of Sb(III) and Sb(V). The effect of carbo ...
... method is based on the selective generation of stibine from Sb(III) in a continuous flow system using atomic fluorescence spectrometry for detection. Sb(V) is masked by citric or oxalic acid in HCl medium. The procedure was optimized with synthetic solutions of Sb(III) and Sb(V). The effect of carbo ...
Syllabus Advanced Level and Advanced Subsidiary Level
... It is intended that candidates should be directed towards the practice of experimental skills throughout the whole period of their course of study. Candidates’ experimental skills will be tested in papers 3 and 5. Paper 3 is a practical examination that will test the skills of manipulation of appara ...
... It is intended that candidates should be directed towards the practice of experimental skills throughout the whole period of their course of study. Candidates’ experimental skills will be tested in papers 3 and 5. Paper 3 is a practical examination that will test the skills of manipulation of appara ...
Document
... law in the absorption peak at 302 nm for DOPA concentrations above 0.3 M, implying DOPA self-association at high concentration. Therefore, 1H NMR data obtained at concentrations above 0.3 M were not used in the determination of the DOPA association constants. Selfassociation was not significant for ...
... law in the absorption peak at 302 nm for DOPA concentrations above 0.3 M, implying DOPA self-association at high concentration. Therefore, 1H NMR data obtained at concentrations above 0.3 M were not used in the determination of the DOPA association constants. Selfassociation was not significant for ...
M. Sc. Semester -I CHE401 Inorganic Chemistry
... Calibration curves, standard addition technique and internal standards. Chemical concentrations. UNIT-3 Fundamentals of Spectrophotometry Properties of light, absorption of light, interaction of light with matter and origin of spectra. The spectrophotometer- calibration, sources of light, monochroma ...
... Calibration curves, standard addition technique and internal standards. Chemical concentrations. UNIT-3 Fundamentals of Spectrophotometry Properties of light, absorption of light, interaction of light with matter and origin of spectra. The spectrophotometer- calibration, sources of light, monochroma ...
conjugate base - DarringtonScience
... Kw= [H+][OH-]=(x)(x) = 1.0x10-14 x2 = 1.0x10-14 x = 1.0x10-7 M = [H+] = [OH-] ...
... Kw= [H+][OH-]=(x)(x) = 1.0x10-14 x2 = 1.0x10-14 x = 1.0x10-7 M = [H+] = [OH-] ...
Exam Review Packet Table of Contents
... and Q2 are the charges in the ions, in CaO these are +2 and -‐2 respectively, while in K2O they are +1 and -‐2. The r (the distance between ions) is slightly smaller in CaO, combined wi ...
... and Q2 are the charges in the ions, in CaO these are +2 and -‐2 respectively, while in K2O they are +1 and -‐2. The r (the distance between ions) is slightly smaller in CaO, combined wi ...
Physical Chemistry 3: — Chemical Kinetics - Christian
... textbooks used in the PC-1 and PC-2 courses. This is done in recognition of the established research focus at the Institute of Physical Chemistry at CAU to enable students to pursue B.Sc. thesis projects in Physical Chemistry. Some more specialized sections have been marked by asterisks and may be o ...
... textbooks used in the PC-1 and PC-2 courses. This is done in recognition of the established research focus at the Institute of Physical Chemistry at CAU to enable students to pursue B.Sc. thesis projects in Physical Chemistry. Some more specialized sections have been marked by asterisks and may be o ...
Now we turn to the study of chemical kinetics. Kinetics is the study of
... What controls the speed of a reaction? One factor is the identity of reactants and products. If one of the reactants is particularly stable, then the speed of the reaction is likely to be slow. If on the other hand a substance is intrinsically unstable, then it will react quickly. A second factor is ...
... What controls the speed of a reaction? One factor is the identity of reactants and products. If one of the reactants is particularly stable, then the speed of the reaction is likely to be slow. If on the other hand a substance is intrinsically unstable, then it will react quickly. A second factor is ...
EIT Review S2012 Part 2 Dr. J. Mack CSUS Department of Chemistry
... equilibrium constants for both aqueous and gaseous systems. • In these cases, the symbol K is sometimes given the subscript “c” for “concentration,” as in Kc. • For gases, however, equilibrium constant expressions can be written in another way: in terms of partial pressures of reactants and pr ...
... equilibrium constants for both aqueous and gaseous systems. • In these cases, the symbol K is sometimes given the subscript “c” for “concentration,” as in Kc. • For gases, however, equilibrium constant expressions can be written in another way: in terms of partial pressures of reactants and pr ...
5.2 Calculations of Enthalpy Changes (SL/HL)
... 5.1 Exothermic and Endothermic Reactions (SL/HL) There are often energy changes that take place during a chemical reaction. These may be in the form of light, sound, but much more commonly heat energy. Reactions that release heat energy are called exothermic reactions. These cause a rise in te ...
... 5.1 Exothermic and Endothermic Reactions (SL/HL) There are often energy changes that take place during a chemical reaction. These may be in the form of light, sound, but much more commonly heat energy. Reactions that release heat energy are called exothermic reactions. These cause a rise in te ...
Solutions
... One of the main difficulties in the study of solutions is the wide variety of variables and units used to specify concentration (in the case of ideal mixtures, only molar fractions, and sometimes mass fractions, were common). Generically speaking, concentrations in a mixture express the quantitative ...
... One of the main difficulties in the study of solutions is the wide variety of variables and units used to specify concentration (in the case of ideal mixtures, only molar fractions, and sometimes mass fractions, were common). Generically speaking, concentrations in a mixture express the quantitative ...
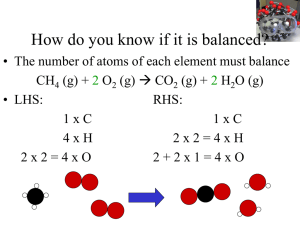
Molecules, Moles and Chemical Equations File
... If you look carefully at the reactions we’ve been writing, you’ll notice more than just the formulas and the states of the substances in the equation. Numerical information about the relative amounts of the substances involved is also given. For example, we included the number “2” in front of both t ...
... If you look carefully at the reactions we’ve been writing, you’ll notice more than just the formulas and the states of the substances in the equation. Numerical information about the relative amounts of the substances involved is also given. For example, we included the number “2” in front of both t ...






![CUCURBIT[7]URIL HOST-GUEST COMPLEXES WITH DRUG MOLECULES CONTAINING ISOQUINOLINE GROUPS Julian Kwok by](http://s1.studyres.com/store/data/008101179_1-fa974bb5e0d463f251947f4fb85d5098-300x300.png)














