Basic thermodynamics` definitions. Units and conversions.
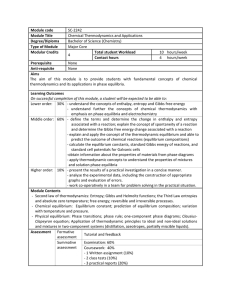
... The objective of the course is to give the idea about the thermodynamic properties such as temperature, pressure, internal energy ,enthalpy, entropy etc. It also deals with the zeroth, first and second Law of Thermodynamics and its applications in environmental engineering field. Thermodynamics help ...
... The objective of the course is to give the idea about the thermodynamic properties such as temperature, pressure, internal energy ,enthalpy, entropy etc. It also deals with the zeroth, first and second Law of Thermodynamics and its applications in environmental engineering field. Thermodynamics help ...
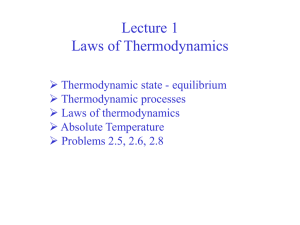
Lecture I
... • Zeroth law of thermodynamics. • Do we need definition of temperature ? • On a cold winters day a metal railing feels much colder than a wooden fence post, but they are both at the same temperature. ...
... • Zeroth law of thermodynamics. • Do we need definition of temperature ? • On a cold winters day a metal railing feels much colder than a wooden fence post, but they are both at the same temperature. ...
• Thermodynamics, what is it? • System, Surrounding and Boundary
... A pure substance can exist in more than one phase, but its chemical composition must be the same in each phase. For example, if liquid water and water vapor form a system with two phases, the system can be regarded as a pure substance because each phase has the same composition. The nature of phases ...
... A pure substance can exist in more than one phase, but its chemical composition must be the same in each phase. For example, if liquid water and water vapor form a system with two phases, the system can be regarded as a pure substance because each phase has the same composition. The nature of phases ...
Lect1.LawsofThr
... macroscopic state of equilibrium. Microscopic state is defined by atomic positions, and momenta - many microscopic states are consistent with a macroscopic state Statistical mechanics connects microscopic description and detail with macroscopic state via ensemble average ...
... macroscopic state of equilibrium. Microscopic state is defined by atomic positions, and momenta - many microscopic states are consistent with a macroscopic state Statistical mechanics connects microscopic description and detail with macroscopic state via ensemble average ...
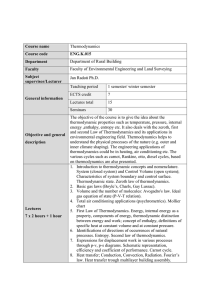
SCH 303: THERMODYNAMICS II AND PHASE EQUILIBRIA Course
... To define systems properties such as U, H, S as path dependent or exact differentials and the consequences. To give thermodynamic determinants of spontaneous process To evaluate the entropy of systems under different conditions To define the Gibbs and Helmhotz free energies and establish their impor ...
... To define systems properties such as U, H, S as path dependent or exact differentials and the consequences. To give thermodynamic determinants of spontaneous process To evaluate the entropy of systems under different conditions To define the Gibbs and Helmhotz free energies and establish their impor ...