Thermo I
... contributions in the fields of statistical mechanics and statistical thermodynamics. ...
... contributions in the fields of statistical mechanics and statistical thermodynamics. ...
648 CHAPTER 17. ELECTRIC POTENTIAL ENERGY AND THE
... Note that since we usually express speeds in the SI units of meters per second, we start this part of the problem with kinetic energy expressed in the SI units of joules. 17.44 The work done by the total force on the electron is equal to the change in its kinetic energy. The electric force is the on ...
... Note that since we usually express speeds in the SI units of meters per second, we start this part of the problem with kinetic energy expressed in the SI units of joules. 17.44 The work done by the total force on the electron is equal to the change in its kinetic energy. The electric force is the on ...
Work-Energy Theorem
... Introduction The Work-Energy Theorem states that the work done on a body by an external force is related to the resulting change in kinetic (K) and/or potential (U) energy. Work = ΔK + ΔU When a spring is disturbed from its equilibrium position, it's potential energy is determined by Us = ½ kx2 wher ...
... Introduction The Work-Energy Theorem states that the work done on a body by an external force is related to the resulting change in kinetic (K) and/or potential (U) energy. Work = ΔK + ΔU When a spring is disturbed from its equilibrium position, it's potential energy is determined by Us = ½ kx2 wher ...
統計力學 1. Consider a binary mixture that consists of n1 moles of
... molecules. It is assumed that the energy ε is required per atom for transforming the solid into separate atoms. For simplicity, take the Einstein model for the vibration of atoms in the solid, i.e. assume that each atom is represented by a three-dimensional harmonic oscillator performing vibration w ...
... molecules. It is assumed that the energy ε is required per atom for transforming the solid into separate atoms. For simplicity, take the Einstein model for the vibration of atoms in the solid, i.e. assume that each atom is represented by a three-dimensional harmonic oscillator performing vibration w ...
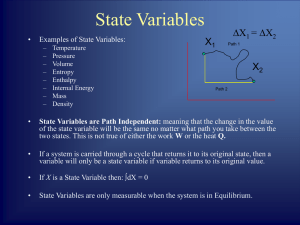
State of the system
... unique for a given state. Clearly definable only for systems in equilibrium. ...
... unique for a given state. Clearly definable only for systems in equilibrium. ...
241 Lecture 11
... • Two systems are said to be in thermal equilibrium if there is no net flow of heat between them when they are brought into thermal contact. • Temperature is the indicator of thermal equilibrium • Two systems individually in thermal equilibrium with a third system are in thermal equilibrium with ...
... • Two systems are said to be in thermal equilibrium if there is no net flow of heat between them when they are brought into thermal contact. • Temperature is the indicator of thermal equilibrium • Two systems individually in thermal equilibrium with a third system are in thermal equilibrium with ...
Thermal and Statistical Physics (Part II) Examples Sheet 1
... observed value of ⟨x2 ⟩ was 3.3×10−12 m2 in a 10-second interval. Use these data to determine a value of the Boltzmann constant, kB , and compare it with the modern value. 31. The famous ratchet and pawl machine, originally suggested by Smoluchowski in 1912 to be able to extract useful work from a t ...
... observed value of ⟨x2 ⟩ was 3.3×10−12 m2 in a 10-second interval. Use these data to determine a value of the Boltzmann constant, kB , and compare it with the modern value. 31. The famous ratchet and pawl machine, originally suggested by Smoluchowski in 1912 to be able to extract useful work from a t ...
MP HW14 solution (due Apr 18st) PHY211 spring 2014
... Explanation1: The satellite and planet form a binary system. In general, for a many body system, the potential energy released in descent from ∞ is shared between the kinetic energies of multiple bodies interacting through force laws. FYI/For the curious: Explanation2: This is a special case of a ge ...
... Explanation1: The satellite and planet form a binary system. In general, for a many body system, the potential energy released in descent from ∞ is shared between the kinetic energies of multiple bodies interacting through force laws. FYI/For the curious: Explanation2: This is a special case of a ge ...
here
... • Entropy: A measure of the extent to which the energy of a system is unavailable. A mathematically defined thermodynamic function of state, the increase in which gives a measure of the energy of a system which has ceased to be available for work during a certain process: ds = (du + pdv)/T >= dq/T w ...
... • Entropy: A measure of the extent to which the energy of a system is unavailable. A mathematically defined thermodynamic function of state, the increase in which gives a measure of the energy of a system which has ceased to be available for work during a certain process: ds = (du + pdv)/T >= dq/T w ...
Equipartition theorem

In classical statistical mechanics, the equipartition theorem is a general formula that relates the temperature of a system with its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition. The original idea of equipartition was that, in thermal equilibrium, energy is shared equally among all of its various forms; for example, the average kinetic energy per degree of freedom in the translational motion of a molecule should equal that of its rotational motions.The equipartition theorem makes quantitative predictions. Like the virial theorem, it gives the total average kinetic and potential energies for a system at a given temperature, from which the system's heat capacity can be computed. However, equipartition also gives the average values of individual components of the energy, such as the kinetic energy of a particular particle or the potential energy of a single spring. For example, it predicts that every atom in a monatomic ideal gas has an average kinetic energy of (3/2)kBT in thermal equilibrium, where kB is the Boltzmann constant and T is the (thermodynamic) temperature. More generally, it can be applied to any classical system in thermal equilibrium, no matter how complicated. The equipartition theorem can be used to derive the ideal gas law, and the Dulong–Petit law for the specific heat capacities of solids. It can also be used to predict the properties of stars, even white dwarfs and neutron stars, since it holds even when relativistic effects are considered.Although the equipartition theorem makes very accurate predictions in certain conditions, it becomes inaccurate when quantum effects are significant, such as at low temperatures. When the thermal energy kBT is smaller than the quantum energy spacing in a particular degree of freedom, the average energy and heat capacity of this degree of freedom are less than the values predicted by equipartition. Such a degree of freedom is said to be ""frozen out"" when the thermal energy is much smaller than this spacing. For example, the heat capacity of a solid decreases at low temperatures as various types of motion become frozen out, rather than remaining constant as predicted by equipartition. Such decreases in heat capacity were among the first signs to physicists of the 19th century that classical physics was incorrect and that a new, more subtle, scientific model was required. Along with other evidence, equipartition's failure to model black-body radiation—also known as the ultraviolet catastrophe—led Max Planck to suggest that energy in the oscillators in an object, which emit light, were quantized, a revolutionary hypothesis that spurred the development of quantum mechanics and quantum field theory.