Chapter 10: Thermodynamics
... balloon expanding slowly during the day. • Adiabatic process: a thermodynamic process during which heat energy is transferred to or from the system. ex: usually a fast process like filling a tank • Isobaric process: a process that takes place at a constant pressure. ex: heating an open pot of water ...
... balloon expanding slowly during the day. • Adiabatic process: a thermodynamic process during which heat energy is transferred to or from the system. ex: usually a fast process like filling a tank • Isobaric process: a process that takes place at a constant pressure. ex: heating an open pot of water ...
15-3 Constant Volume and Constant Pressure Processes
... substance of mass m and specific heat c. For gases, a more convenient equation is ...
... substance of mass m and specific heat c. For gases, a more convenient equation is ...
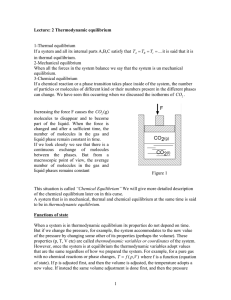
1 Lecture: 2 Thermodynamic equilibrium 1
... constant for a conservative system. If we include all the variables that describe the processes, all systems are conservative. It follows that the energy is always conserved. We consider a system “A” surrounded by the rest of the universe, and we say that the system has a certain amount of energy U ...
... constant for a conservative system. If we include all the variables that describe the processes, all systems are conservative. It follows that the energy is always conserved. We consider a system “A” surrounded by the rest of the universe, and we say that the system has a certain amount of energy U ...
The Second Law of Thermodynamics
... The reciprocal of the absolute temperature is an integrating factor that permits the replacement of the inexact differential by the exact differential . The above equation is the Clausius definition of the entropy S. The first law of thermodynamics can be now expressed as for a reversible process ...
... The reciprocal of the absolute temperature is an integrating factor that permits the replacement of the inexact differential by the exact differential . The above equation is the Clausius definition of the entropy S. The first law of thermodynamics can be now expressed as for a reversible process ...
Model Question Paper – 1
... volume of 0.22 m3 to a final pressure of 100 kPa in a process described by PV1.2 = constant. a) If expansion is quasi-static, find heat transfer, work transfer and change in internal energy. b) In another process the same system expands according to the same P-v relation as in part (a); and between ...
... volume of 0.22 m3 to a final pressure of 100 kPa in a process described by PV1.2 = constant. a) If expansion is quasi-static, find heat transfer, work transfer and change in internal energy. b) In another process the same system expands according to the same P-v relation as in part (a); and between ...
PHYS 1220, Engineering Physics, Chapter 19 – The First Law of
... • A status is to describe the states of a system. It does not depend on the history of the system, rather it only depends on its thermodynamic state (e.g. temperature, volume, pressure, number of molecules etc.) - What is a “process”? • A process is to describe how the system evolve from one state ( ...
... • A status is to describe the states of a system. It does not depend on the history of the system, rather it only depends on its thermodynamic state (e.g. temperature, volume, pressure, number of molecules etc.) - What is a “process”? • A process is to describe how the system evolve from one state ( ...
p250c13
... 10ºC. What is the theoretical maximum cp for this heat pump? If the pump is to deliver heat at a rate of 15 kW, how much power must be supplied to run the pump? ...
... 10ºC. What is the theoretical maximum cp for this heat pump? If the pump is to deliver heat at a rate of 15 kW, how much power must be supplied to run the pump? ...
Course name Thermodynamics Course code ENG.I.011 Department
... air conditioning etc. The various cycles such as carnot, Rankine, otto, diesel cycles, based on thermodynamics are also presented. 1. Introduction to thermodynamic concepts and nomenclature. System (closed system) and Control Volume (open system); Characteristics of system boundary and control surfa ...
... air conditioning etc. The various cycles such as carnot, Rankine, otto, diesel cycles, based on thermodynamics are also presented. 1. Introduction to thermodynamic concepts and nomenclature. System (closed system) and Control Volume (open system); Characteristics of system boundary and control surfa ...
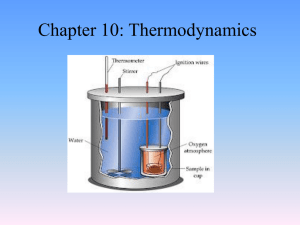
Calorimetry

Calorimetry is the science or act of measuring changes in state variables of a body for the purpose of deriving the heat transfer associated with changes of its state due for example to chemical reactions, physical changes, or phase transitions under specified constraints. Calorimetry is performed with a calorimeter. The word calorimetry is derived from the Latin word calor, meaning heat and the Greek word μέτρον (metron), meaning measure. Scottish physician and scientist Joseph Black, who was the first to recognize the distinction between heat and temperature, is said to be the founder of the science of calorimetry.Indirect Calorimetry calculates heat that living organisms produce by measuring either their production of carbon dioxide and nitrogen waste (frequently ammonia in aquatic organisms, or urea in terrestrial ones), or from their consumption of oxygen. Lavoisier noted in 1780 that heat production can be predicted from oxygen consumption this way, using multiple regression. The Dynamic Energy Budget theory explains why this procedure is correct. Heat generated by living organisms may also be measured by direct calorimetry, in which the entire organism is placed inside the calorimeter for the measurement.A widely used modern instrument is the differential scanning calorimeter, a device which allows thermal data to be obtained on small amounts of material. It involves heating the sample at a controlled rate and recording the heat flow either into or from the specimen.