“solar system” model of the atom
... 31-1 Early Models of the Atom The electron was discovered in 1897, and was observed to be much smaller than the atom. It was known that atoms are electrically neutral; the first modern model of the atom was therefore the “plum pudding” model – tiny electrons embedded in a mass of positive charge. ...
... 31-1 Early Models of the Atom The electron was discovered in 1897, and was observed to be much smaller than the atom. It was known that atoms are electrically neutral; the first modern model of the atom was therefore the “plum pudding” model – tiny electrons embedded in a mass of positive charge. ...
NM Strand
... 50. A student spills a chemical in the laboratory. What should he do first? 51. A sour candy has a pH of: 52. A characteristic that can be observed or measured without changing the sample’s composition is 53. An experiment that determines the maximum number of grams of a substance that will dissolve ...
... 50. A student spills a chemical in the laboratory. What should he do first? 51. A sour candy has a pH of: 52. A characteristic that can be observed or measured without changing the sample’s composition is 53. An experiment that determines the maximum number of grams of a substance that will dissolve ...
Ch02-sample-and-practice-set-2
... (a) The number of protons (22) is the atomic number of the element. By referring to a periodic table or list of elements, we see that the element with atomic number 22 is titanium (Ti). The mass number of this isotope of titanium is 22 + 26 = 48 (the sum of the protons and neutrons). Because the ion ...
... (a) The number of protons (22) is the atomic number of the element. By referring to a periodic table or list of elements, we see that the element with atomic number 22 is titanium (Ti). The mass number of this isotope of titanium is 22 + 26 = 48 (the sum of the protons and neutrons). Because the ion ...
Quantum Theory - akugakbutuheksis
... No electrons were emitted until the frequency of the light exceeded a critical frequency, at which point electrons were emitted from the surface! (Recall: small l large n) ...
... No electrons were emitted until the frequency of the light exceeded a critical frequency, at which point electrons were emitted from the surface! (Recall: small l large n) ...
CP Chemistry Final Exam Review Sheet
... 50. What is the octet rule? The octet rule states that atoms will gain, lose, or share electrons in order to get a full octet (8 e-) in the valence (outermost) shell of an atom. 51. An ion is a particle with an electrical charge created by the transfer (loss or gaining) of electrons. 52. What is a c ...
... 50. What is the octet rule? The octet rule states that atoms will gain, lose, or share electrons in order to get a full octet (8 e-) in the valence (outermost) shell of an atom. 51. An ion is a particle with an electrical charge created by the transfer (loss or gaining) of electrons. 52. What is a c ...
B.R. Martin. Nuclear and Particle Physics. Appendix A. Some results
... In 1909 they observed that alpha particles from radioactive decays occasionally scatter at angles greater than 90°, which is physically impossible unless they are scattering off something more massive than themselves. This led Rutherford to deduce that the positive charge in an atom is concentrated ...
... In 1909 they observed that alpha particles from radioactive decays occasionally scatter at angles greater than 90°, which is physically impossible unless they are scattering off something more massive than themselves. This led Rutherford to deduce that the positive charge in an atom is concentrated ...
Chemistry II Demonstration Assessment
... To observe a decomposition chemical reaction involving the formation of the constituent elements from a compound. To use electrical energy to break the chemical bonds of a compound. Background Information: Chemical reactions occur when atoms are separated, rearranged, and/or joined in a new way. In ...
... To observe a decomposition chemical reaction involving the formation of the constituent elements from a compound. To use electrical energy to break the chemical bonds of a compound. Background Information: Chemical reactions occur when atoms are separated, rearranged, and/or joined in a new way. In ...
chemia simr01 en - Leszek Niedzicki
... +i• If positive charge is constant (nucleus) substracting electrons (anion forming) decreases ionic radius (positive charge attraction is constant) – electrons number is decreasing – they repel each other less – so they can fit in a smaller volume. Adding electrons (cation forming) increases ionic r ...
... +i• If positive charge is constant (nucleus) substracting electrons (anion forming) decreases ionic radius (positive charge attraction is constant) – electrons number is decreasing – they repel each other less – so they can fit in a smaller volume. Adding electrons (cation forming) increases ionic r ...
Chapter 2 Practice Questions
... B) A given compound always contains exactly the same proportion of elements by mass. C) When two elements form a series of compounds, the ratios of masses that combine with 1 gram of the first element can always be reduced to small whole numbers. D) At the same temperature and pressure, equal volume ...
... B) A given compound always contains exactly the same proportion of elements by mass. C) When two elements form a series of compounds, the ratios of masses that combine with 1 gram of the first element can always be reduced to small whole numbers. D) At the same temperature and pressure, equal volume ...
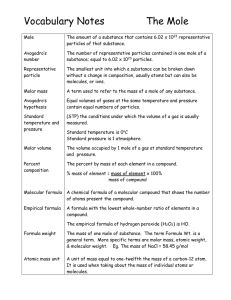
Vocabulary Notes
... The mass of one mole of substance. The term Formula Wt. is a general term. More specific terms are molar mass, atomic weight, & molecular weight. Eg. The mass of NaCl = 58.45 g/mol ...
... The mass of one mole of substance. The term Formula Wt. is a general term. More specific terms are molar mass, atomic weight, & molecular weight. Eg. The mass of NaCl = 58.45 g/mol ...
Hinge Point Questions
... which cancel out. c) The neutrons make them neutral. d) Atoms have the same number of protons and electrons which cancel out. ...
... which cancel out. c) The neutrons make them neutral. d) Atoms have the same number of protons and electrons which cancel out. ...
200 Ways to Pass the Chemistry
... 16. The Bohr Model of the atom placed electrons in “planet-like” orbits around the nucleus of an atom. 17. The current wave-mechanical model of the atom has electrons in “clouds” (orbitals) around the nucleus. 18. Electrons can be excited to jump to higher energy levels. They emit energy as light wh ...
... 16. The Bohr Model of the atom placed electrons in “planet-like” orbits around the nucleus of an atom. 17. The current wave-mechanical model of the atom has electrons in “clouds” (orbitals) around the nucleus. 18. Electrons can be excited to jump to higher energy levels. They emit energy as light wh ...
Mn2 1 Many-particle Systems, 2 Multi
... To see how the Pauli Exclusion Principle produces atomic diversity, it is useful to begin simply, in particular, by considering the most elementary multi-electron “atom”: the hydrogen anion, H–. H– is a system of one proton and two electrons. Suppose the two electrons interact only with the proton a ...
... To see how the Pauli Exclusion Principle produces atomic diversity, it is useful to begin simply, in particular, by considering the most elementary multi-electron “atom”: the hydrogen anion, H–. H– is a system of one proton and two electrons. Suppose the two electrons interact only with the proton a ...
Coulomb Explosion Imaging - ultrafast dynamic imaging 2009
... •The electron direction determines the field direction at the moment of ionization •The bond softened ion determines the molecule’s direction at the moment of ionization ...
... •The electron direction determines the field direction at the moment of ionization •The bond softened ion determines the molecule’s direction at the moment of ionization ...
Zero energy non-zero momentum particles
... In the article with title “Matter-light duality and speed greater than light” [1], we formulated energy and momentum equations for particles with speed greater than light. In this article we see a special case of it where the energy becomes zero and momentum is non-zero for a particle (we named them ...
... In the article with title “Matter-light duality and speed greater than light” [1], we formulated energy and momentum equations for particles with speed greater than light. In this article we see a special case of it where the energy becomes zero and momentum is non-zero for a particle (we named them ...
File - Lenora Henderson`s Flipped Chemistry Classroom
... to find an electron in a particular area surrounding the nucleus The probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloudlike region The cloud is more dense where there is a high probability of finding an electron, and less d ...
... to find an electron in a particular area surrounding the nucleus The probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloudlike region The cloud is more dense where there is a high probability of finding an electron, and less d ...
484
... The phoron flux emittance ;~ppearsto be the quadratic sum of the particle beam cmittance and of the product 0.0’. Since G is the source si7.c. and ci’ the minimum divergence, (SCT’can he described as the intrinsic emittance of the photons. A f+oposed Definition of “Low” Emittance ‘I‘hc formula ilO’) ...
... The phoron flux emittance ;~ppearsto be the quadratic sum of the particle beam cmittance and of the product 0.0’. Since G is the source si7.c. and ci’ the minimum divergence, (SCT’can he described as the intrinsic emittance of the photons. A f+oposed Definition of “Low” Emittance ‘I‘hc formula ilO’) ...
Question 2
... In all questions show all relevant working and balance any equations you write. 1. Electrolytes & non-electrolytes Indicate if you would expect the following compounds to be electrolytes or non-electrolytes when in aqueous solution. In each case very briefly explain your answer. Use equations if app ...
... In all questions show all relevant working and balance any equations you write. 1. Electrolytes & non-electrolytes Indicate if you would expect the following compounds to be electrolytes or non-electrolytes when in aqueous solution. In each case very briefly explain your answer. Use equations if app ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.