From Last Time… - High Energy Physics
... ( x ) ( p ) is always greater than ( h / 4 ) Often write this as where ...
... ( x ) ( p ) is always greater than ( h / 4 ) Often write this as where ...
atomic theory of matter
... Compounds with Polyatomic ions • Name these the same way that ionic binary compounds are named replacing the name of the anion (in a few cases cation) with the name of the polyatomic ion. – Oxy anions (anions containing at least 1 oxygen atom): difficult because there are often several possible ani ...
... Compounds with Polyatomic ions • Name these the same way that ionic binary compounds are named replacing the name of the anion (in a few cases cation) with the name of the polyatomic ion. – Oxy anions (anions containing at least 1 oxygen atom): difficult because there are often several possible ani ...
Chapter 1 Glossary The Nature of Chemistry
... The intermolecular attraction between the partial negative end of one polar molecule and the partial positive end of another polar molecule. Hydrogen bond The intermolecular attraction between a nitrogen, oxygen, or fluorine atom of one molecule and a hydrogen atom bonded to a nitrogen, oxygen, or f ...
... The intermolecular attraction between the partial negative end of one polar molecule and the partial positive end of another polar molecule. Hydrogen bond The intermolecular attraction between a nitrogen, oxygen, or fluorine atom of one molecule and a hydrogen atom bonded to a nitrogen, oxygen, or f ...
6 Electronic Structure of Atoms
... diffuse negative charge (electrons) surrounding it. Bohr’s theory then specified the nature of the diffuse negative charge. The prevailing theory before the nuclear model was Thomson’s plum pudding or watermelon model, with discrete electrons scattered about a diffuse positive charge cloud. Bohr’s t ...
... diffuse negative charge (electrons) surrounding it. Bohr’s theory then specified the nature of the diffuse negative charge. The prevailing theory before the nuclear model was Thomson’s plum pudding or watermelon model, with discrete electrons scattered about a diffuse positive charge cloud. Bohr’s t ...
Geometrical Optics
... For each of your measurements separately, calculate the index of refraction of the plastic material from Snell's law, then average your calculated values to get the mean value and determine the standard deviation and the standard deviation of the mean. You will need to have a good sketch of your set ...
... For each of your measurements separately, calculate the index of refraction of the plastic material from Snell's law, then average your calculated values to get the mean value and determine the standard deviation and the standard deviation of the mean. You will need to have a good sketch of your set ...
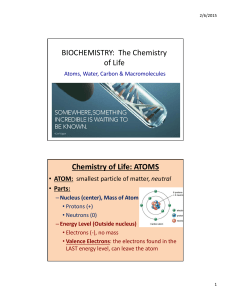
BIOCHEMISTRY: The Chemistry of Life Chemistry of Life: ATOMS
... Chemistry of Life: ATOMS • Atomic #: –# of Protons in an atom –Order on the Periodic Table of Elements • Atomic Mass: –Sum of the Protons & Neutrons in the nucleus of the Atom – Atomic Mass – Atomic # = # of Neutrons ...
... Chemistry of Life: ATOMS • Atomic #: –# of Protons in an atom –Order on the Periodic Table of Elements • Atomic Mass: –Sum of the Protons & Neutrons in the nucleus of the Atom – Atomic Mass – Atomic # = # of Neutrons ...
IPC Semester Exam Review – Chemistry Topics
... heterogeneous mixtures that scatter light, whereas solutions are homogeneous mixtures that don’t scatter light. Solutions have the smallest particles, suspensions have the largest particles. 41. physical 42. chemical 43. chemical 44. physical 45. chemical 46. physical 47. physical 48. chemical 49. B ...
... heterogeneous mixtures that scatter light, whereas solutions are homogeneous mixtures that don’t scatter light. Solutions have the smallest particles, suspensions have the largest particles. 41. physical 42. chemical 43. chemical 44. physical 45. chemical 46. physical 47. physical 48. chemical 49. B ...
EXCITATION OF HELIUM ATOMS IN He
... mentioned levels of transitions between the g–g, g–g–g and other states of a quasimolecule. The polarization degree at the excitation of the level with spin, which is not equal zero, is not significant because the angular distribution of photons “are smeared” because of presence of the spin-orb ...
... mentioned levels of transitions between the g–g, g–g–g and other states of a quasimolecule. The polarization degree at the excitation of the level with spin, which is not equal zero, is not significant because the angular distribution of photons “are smeared” because of presence of the spin-orb ...
class slides for Chapter 38
... up on the screen, we can speculate that each photon travels from source to screen as a wave that fills up the space between source and screen and then vanishes in a photon absorption at some point on the screen, with a transfer of energy and momentum to the screen at that point. We cannot predict wh ...
... up on the screen, we can speculate that each photon travels from source to screen as a wave that fills up the space between source and screen and then vanishes in a photon absorption at some point on the screen, with a transfer of energy and momentum to the screen at that point. We cannot predict wh ...
section_3.2
... To understand and illustrate the Law of constant composition To learn how a formula describes a compound’s composition ...
... To understand and illustrate the Law of constant composition To learn how a formula describes a compound’s composition ...
Thermochemistry Unit Review - WilsonSCH4U-03-2012
... (note: In problems where it is necessary to report the final temperature, add or subtract t from initial temperature depending on whether reaction is endothermic or exothermic) For drill problems using this calculation, go to http://science.widener.edu/svb/tutorial/waterheatcsn7.html Kinetic theory ...
... (note: In problems where it is necessary to report the final temperature, add or subtract t from initial temperature depending on whether reaction is endothermic or exothermic) For drill problems using this calculation, go to http://science.widener.edu/svb/tutorial/waterheatcsn7.html Kinetic theory ...
Lectures 10-11: Multi-electron atoms System of non
... o Exchange energy is sometimes written in the form "E exchange = #2J$% Sˆ1 & Sˆ 2 which shows explicitly that the change of energy is related to the relative alignment of the electron spins. If aligned = > energy goes up. ...
... o Exchange energy is sometimes written in the form "E exchange = #2J$% Sˆ1 & Sˆ 2 which shows explicitly that the change of energy is related to the relative alignment of the electron spins. If aligned = > energy goes up. ...
Chemistry 11 Review Sheet
... Describe electrolysis and distillation and other methods that can be used to separate mixtures? Draw the classification of matter chart and define and give examples of elements, compounds, ions atoms, molecules and polyatomic atoms, diatomic atoms and metal and nonmetals. Explain the law of definite ...
... Describe electrolysis and distillation and other methods that can be used to separate mixtures? Draw the classification of matter chart and define and give examples of elements, compounds, ions atoms, molecules and polyatomic atoms, diatomic atoms and metal and nonmetals. Explain the law of definite ...
Atoms: Some Basics
... not know n, using the closest larger integer than n∗ still leads to useful results), remains very constant with respect to n, but decreases rapidly with respect to L. A more accurate empirical formula for the term values of a series is the Balmer-Ritz formula ...
... not know n, using the closest larger integer than n∗ still leads to useful results), remains very constant with respect to n, but decreases rapidly with respect to L. A more accurate empirical formula for the term values of a series is the Balmer-Ritz formula ...
introduction - Stanford Synchrotron Radiation Lightsource
... Two experimental halls are provided, one close to the undulator exit (Hall A, starting ~ 50 m from the undulator end) and one downstream of the undulator (Hall B, starting ~ 400 m from the undulator end). The total experimental floor area is a requirement of the first 6 experiments planned (includin ...
... Two experimental halls are provided, one close to the undulator exit (Hall A, starting ~ 50 m from the undulator end) and one downstream of the undulator (Hall B, starting ~ 400 m from the undulator end). The total experimental floor area is a requirement of the first 6 experiments planned (includin ...
Quantum eraser
... unitary operator. In order to get a more intuitive notion of the reason for this, we will further simplify the model by mapping it to a particle (the quanta) in 3 sites system (A, B, D). In this model the hamiltonian Hi couples site i to the D site. So using both of the hamiltonians together will ca ...
... unitary operator. In order to get a more intuitive notion of the reason for this, we will further simplify the model by mapping it to a particle (the quanta) in 3 sites system (A, B, D). In this model the hamiltonian Hi couples site i to the D site. So using both of the hamiltonians together will ca ...
Quantum Statistical Mechanics Initial questions: What holds up
... Thermodynamic and chemical equilibrium require that if there are reactions that might P change the Ni , then i µi dNi = 0. Photon number is not conserved, so µγ = 0. Note that here we’re interested in reactions that take place fairly rapidly, so things like nuclear reactions (which usually take year ...
... Thermodynamic and chemical equilibrium require that if there are reactions that might P change the Ni , then i µi dNi = 0. Photon number is not conserved, so µγ = 0. Note that here we’re interested in reactions that take place fairly rapidly, so things like nuclear reactions (which usually take year ...
Atomic Masses
... The most accurate method of comparing masses of atoms is by using the mass spectrometer. The mass spectrometer knocks electrons off the atoms or molecules being analyzed and changes them into positive ions. An applied electric field then accelerates these ions into a magnetic field. An accelerating ...
... The most accurate method of comparing masses of atoms is by using the mass spectrometer. The mass spectrometer knocks electrons off the atoms or molecules being analyzed and changes them into positive ions. An applied electric field then accelerates these ions into a magnetic field. An accelerating ...
2016 Pre Course CHEMISTRY - Calday Grange Grammar School
... An atom of element Z has two more protons and two more neutrons than an atom of Give the symbol, including mass number and atomic number, for this atom of Z. ...
... An atom of element Z has two more protons and two more neutrons than an atom of Give the symbol, including mass number and atomic number, for this atom of Z. ...
Chapter 9: Electrons in Atoms
... When no field is present, all ml values have the same energy and both ms values have the same energy. Together the quantum # n, l, ml define an atomic orbital; the quantum number ms describes the electron spin within the orbital. The Multielectron Atoms H atom is the only atom for which Shrödinger e ...
... When no field is present, all ml values have the same energy and both ms values have the same energy. Together the quantum # n, l, ml define an atomic orbital; the quantum number ms describes the electron spin within the orbital. The Multielectron Atoms H atom is the only atom for which Shrödinger e ...
The Infinite Square Well 6.1 Separability of Schrödinger`s Equation
... measurement does. What a measurement is is another question – if you think about it, though, most measurements involve interaction. Those interactions can be carried out using other particles (so we have multiple particles) or turning on and off various detection equipment that measures properties o ...
... measurement does. What a measurement is is another question – if you think about it, though, most measurements involve interaction. Those interactions can be carried out using other particles (so we have multiple particles) or turning on and off various detection equipment that measures properties o ...
Lecture 7 - UIC Department of Chemistry
... Your TA will send the grade (Final & Overall) by the end of Dec 13 TA will send the grade (Final & Overall) by the end of Dec 13 by e‐mail. If you need any corrections, please claim it before Dec 14 at 3 pm. ■ We will return your graded notebook on the day of the Final. ■ We will not return th ...
... Your TA will send the grade (Final & Overall) by the end of Dec 13 TA will send the grade (Final & Overall) by the end of Dec 13 by e‐mail. If you need any corrections, please claim it before Dec 14 at 3 pm. ■ We will return your graded notebook on the day of the Final. ■ We will not return th ...
Chapter 7:The Quantum-Mechanical Model of
... small negatively charged particles inside a divisible atom and came up with the Plum Pudding Model of the atom. Rutherford came up with the gold foil experiment shooting alpha particles through thin gold foil to test the Plum Pudding Model and discovered that some alpha particles were deflected. Thi ...
... small negatively charged particles inside a divisible atom and came up with the Plum Pudding Model of the atom. Rutherford came up with the gold foil experiment shooting alpha particles through thin gold foil to test the Plum Pudding Model and discovered that some alpha particles were deflected. Thi ...
PHYS-201 LAB-03 Bohr`s Model and Emission Spectra of Hydrogen
... mechanics, will lose energy by radiation and would spiral down and eventually fall into the nucleus. Bohr knew this but, since atoms are stable, he also recognized that it was ...
... mechanics, will lose energy by radiation and would spiral down and eventually fall into the nucleus. Bohr knew this but, since atoms are stable, he also recognized that it was ...
Quiz 8
... **4. (2.5pts) A crown glass block (n = 1.520) rests on the bottom of a water tank. The block has a height of 20.0 cm and covered with water (n = 1.333) to a depth of 15.0 cm. What is the apparent height of the block as it is seen from above the water? ...
... **4. (2.5pts) A crown glass block (n = 1.520) rests on the bottom of a water tank. The block has a height of 20.0 cm and covered with water (n = 1.333) to a depth of 15.0 cm. What is the apparent height of the block as it is seen from above the water? ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.