Paper
... the explored physics was governed by mean-field interactions, conveniently described by the Gross-Pitaevskii equation. One novel feature of trapped inhomogeneous gases was the spatially varying density, that allowed for the direct observation of the condensate, but also led to new concepts of surfac ...
... the explored physics was governed by mean-field interactions, conveniently described by the Gross-Pitaevskii equation. One novel feature of trapped inhomogeneous gases was the spatially varying density, that allowed for the direct observation of the condensate, but also led to new concepts of surfac ...
Photoelectron Spectroscopy of SO3 at 355 and 266 nm
... To obtain more reliable energies, additional single point energy calculations were carried out for all of the species using the CCSD(T) (coupled-cluster with all single and double substitutions, and a quasi-perturbative estimate for the effect of connected triple excitations)36-40 method with the cc ...
... To obtain more reliable energies, additional single point energy calculations were carried out for all of the species using the CCSD(T) (coupled-cluster with all single and double substitutions, and a quasi-perturbative estimate for the effect of connected triple excitations)36-40 method with the cc ...
Oxidation numbers
... So the oxidation number of oxygen is −1 in H2O2. (Note that this confirms what has been stated about peroxides (see rule 5).) Oxygen has an oxidation number of −2 in H2O and −1 in H2O2. 5. In the compound NaH, the sum of the oxidation numbers must be 0 (rule 3). This compound is a metal hydride, so ...
... So the oxidation number of oxygen is −1 in H2O2. (Note that this confirms what has been stated about peroxides (see rule 5).) Oxygen has an oxidation number of −2 in H2O and −1 in H2O2. 5. In the compound NaH, the sum of the oxidation numbers must be 0 (rule 3). This compound is a metal hydride, so ...
Mead Chemistry Lap 11: Stoichiometry Chapter 12 12.1 Balanced
... • Number of moles ▫ Use coefficients to find # of moles ▫ __ mole of N2 reacts with __ moles of H2 to form __ moles of NH3 ▫ Ratio of 1 N2: 3 H2 :2 NH3 • Mass in grams ▫ All reactions must follow law of conservation of mass ▫ Atoms and mass will always follow conservation ▫ Use mole ratios and molar ...
... • Number of moles ▫ Use coefficients to find # of moles ▫ __ mole of N2 reacts with __ moles of H2 to form __ moles of NH3 ▫ Ratio of 1 N2: 3 H2 :2 NH3 • Mass in grams ▫ All reactions must follow law of conservation of mass ▫ Atoms and mass will always follow conservation ▫ Use mole ratios and molar ...
C273/SQP365 NATIONAL QUALIFICATIONS Chemistry
... 3 Rough work, if any should be necessary, should be written in this book and then scored through when the fair copy has been written. 4 Additional space for answers and rough work will be found at the end of the book. If further space is required, supplementary sheets may be obtained from the Invigi ...
... 3 Rough work, if any should be necessary, should be written in this book and then scored through when the fair copy has been written. 4 Additional space for answers and rough work will be found at the end of the book. If further space is required, supplementary sheets may be obtained from the Invigi ...
Now! - Soojeede.com
... Acids are a special group of compounds because it has been found that they have their own set of properties. This helps to identify them from other compounds. Thus, if you had a number of compounds that you were wondering whether these were acids or otherwise, you could identify them by their proper ...
... Acids are a special group of compounds because it has been found that they have their own set of properties. This helps to identify them from other compounds. Thus, if you had a number of compounds that you were wondering whether these were acids or otherwise, you could identify them by their proper ...
A pulsed dye laser for photo-ionization of magnesium atoms
... (other species can be ionized and trapped with the desired ions). This problem is avoided when a resonant multi-step photo-ionization process is used. The atoms are rst selectively excited on resonant transitions and then ionized. Hence, only the desired species will have a signi cant ionization pr ...
... (other species can be ionized and trapped with the desired ions). This problem is avoided when a resonant multi-step photo-ionization process is used. The atoms are rst selectively excited on resonant transitions and then ionized. Hence, only the desired species will have a signi cant ionization pr ...
mole - hrsbstaff.ednet.ns.ca
... Chapter 10 - How does a chemist measure? How does the chemist count? 2H2(g) + O2 → 2H2O(g) This reaction requires two molecules of hydrogen and one molecule of oxygen. To carry out this chemical reaction a chemist must mix hydrogen and oxygen together in the correct ratio (2:1). How does the chemist ...
... Chapter 10 - How does a chemist measure? How does the chemist count? 2H2(g) + O2 → 2H2O(g) This reaction requires two molecules of hydrogen and one molecule of oxygen. To carry out this chemical reaction a chemist must mix hydrogen and oxygen together in the correct ratio (2:1). How does the chemist ...
THE ROLE OF FeSO4 IN THE OBTAINING OF
... mechanical properties compared with homopolymers [8]. However, only part of polyvinylpirrolidone participates in the grafted polymerization. Unreacted PVP may be washed out during hydration that affects physicomechanical properties of copolymers. Therefore from the scientific and practical points of ...
... mechanical properties compared with homopolymers [8]. However, only part of polyvinylpirrolidone participates in the grafted polymerization. Unreacted PVP may be washed out during hydration that affects physicomechanical properties of copolymers. Therefore from the scientific and practical points of ...
Plasmon Enhanced Fluorescence (PEF) of High and Low Quantum
... enhance optical signals: Plasmon enhanced fluorescence (PEF). In the literature it has grown with two different names: surface enhanced fluorescence (SEF) and also metal enhanced fluorescence (MEF). In this thesis, we have explored some of the peculiar properties of plasmon enhanced fluorescence. In ...
... enhance optical signals: Plasmon enhanced fluorescence (PEF). In the literature it has grown with two different names: surface enhanced fluorescence (SEF) and also metal enhanced fluorescence (MEF). In this thesis, we have explored some of the peculiar properties of plasmon enhanced fluorescence. In ...
Disproportionation of Gold(II)
... redox data for Au2+ species plus the crudity of our solvation model conspire to forestall quantitative comparisons, the calculations lead to the reasonable supposition that the greater favorability of Au2+ disproportionation in water is driven by the large solvation energy of the 3+ gold ion: PCM ca ...
... redox data for Au2+ species plus the crudity of our solvation model conspire to forestall quantitative comparisons, the calculations lead to the reasonable supposition that the greater favorability of Au2+ disproportionation in water is driven by the large solvation energy of the 3+ gold ion: PCM ca ...
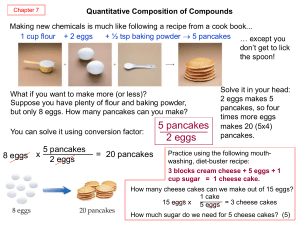
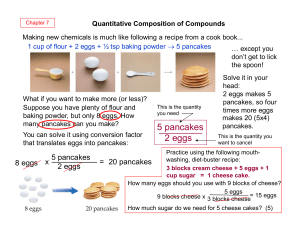
5 pancakes 2 eggs
... 15. 1 molecule of tetraphosphorus decoxide contains: how many moles? How many grams? How many P atoms? How many O atoms? How many total atoms? 26. A 7.52 g sample of ajoene (garlic odor) was found to contain 3.09 g S, 0.453 g H, 0.513 g O and the rest, C. Calculate the percent composition. 39. Ethan ...
... 15. 1 molecule of tetraphosphorus decoxide contains: how many moles? How many grams? How many P atoms? How many O atoms? How many total atoms? 26. A 7.52 g sample of ajoene (garlic odor) was found to contain 3.09 g S, 0.453 g H, 0.513 g O and the rest, C. Calculate the percent composition. 39. Ethan ...
Exam Review Packet Table of Contents
... b) two points -‐ The chloride ion is larger than the chlorine atom because: (i) electron-‐ electron repulsion increases (or shielding increases or the electron-‐proton ratio increases or the effective nucl ...
... b) two points -‐ The chloride ion is larger than the chlorine atom because: (i) electron-‐ electron repulsion increases (or shielding increases or the electron-‐proton ratio increases or the effective nucl ...
From Molecules to Cooper Pairs: Experiments in the BEC
... wave packets of the particles begin to overlap. For bosons this marks the onset of BoseEinstein condensation (BEC). This phase transition into the superfluid condensate is characterized by a macroscopic occupation of the ground state. Identical fermions are far less social and have to occupy differe ...
... wave packets of the particles begin to overlap. For bosons this marks the onset of BoseEinstein condensation (BEC). This phase transition into the superfluid condensate is characterized by a macroscopic occupation of the ground state. Identical fermions are far less social and have to occupy differe ...
DOE Chemistry 1
... for use by DOE category A reactors. The subject areas, subject matter content, and level of detail of the Reactor Operator Fundamentals Manuals were determined from several sources. DOE Category A reactor training managers determined which materials should be included, and served as a primary refere ...
... for use by DOE category A reactors. The subject areas, subject matter content, and level of detail of the Reactor Operator Fundamentals Manuals were determined from several sources. DOE Category A reactor training managers determined which materials should be included, and served as a primary refere ...
Document
... 1. Write and balance equation. 2. Calculate the number of moles of product for each reactant; 3. The reactant that gives the least moles of (the same!) product is the limiting reactant. 4. Find the amount of reactant in excess needed to react with the limiting reactant. Subtract this amount from the ...
... 1. Write and balance equation. 2. Calculate the number of moles of product for each reactant; 3. The reactant that gives the least moles of (the same!) product is the limiting reactant. 4. Find the amount of reactant in excess needed to react with the limiting reactant. Subtract this amount from the ...
CO2 Dissociation using the Versatile Atmospheric Dielectric Barrier
... As of 2013, the Carbon Dioxide Information Analysis Center (CDIAC) estimates that the world emits approximately 36 trillion metric tons of Carbon Dioxide (CO2 ) into the atmosphere every year [1]. These large emissions have been correlated to global warming trends that have many consequences across ...
... As of 2013, the Carbon Dioxide Information Analysis Center (CDIAC) estimates that the world emits approximately 36 trillion metric tons of Carbon Dioxide (CO2 ) into the atmosphere every year [1]. These large emissions have been correlated to global warming trends that have many consequences across ...
BASIS SET SUPERPOSITION ERROR EFFECTS, EXCITED-STATE POTENTIAL ENERGY SURFACE AND
... second order (MP2) when used with some of Pople’s basis sets, can not describe the planar structure of benzene. In addition, if a planar stationary point is optimized, the frequency analysis shows one or more imaginary frequencies. Given that thymine is a planar aromatic molecule as benzene, a bench ...
... second order (MP2) when used with some of Pople’s basis sets, can not describe the planar structure of benzene. In addition, if a planar stationary point is optimized, the frequency analysis shows one or more imaginary frequencies. Given that thymine is a planar aromatic molecule as benzene, a bench ...
Physical Science Standards
... 2.2 explore matter in terms of specific properties . Performance Indicators State (SPI) and Teacher (TPI): At Level 1, the student is able to SPI distinguish among the phases of matter in terms of volume, shape, and particle arrangement, given illustrations. TPI describe and illustrate the differenc ...
... 2.2 explore matter in terms of specific properties . Performance Indicators State (SPI) and Teacher (TPI): At Level 1, the student is able to SPI distinguish among the phases of matter in terms of volume, shape, and particle arrangement, given illustrations. TPI describe and illustrate the differenc ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.















![mclintock.ch6 [Compatibility Mode]](http://s1.studyres.com/store/data/003971396_1-780a12aa3165c9221aca3ac594a06674-300x300.png)