Energy Notes
... • States that if a series of reactions are added together, the enthalpy change for the net reaction will be the sum of the enthalpy changes for the individual steps. • In other words, in going from a particular set of reactants to a particular set of products, the change in enthalpy is the same whet ...
... • States that if a series of reactions are added together, the enthalpy change for the net reaction will be the sum of the enthalpy changes for the individual steps. • In other words, in going from a particular set of reactants to a particular set of products, the change in enthalpy is the same whet ...
LaurenHill Chemistry 534
... closer to each other’s nucleus. The separation distance, d, is small, just like the height in the physics analogy. To break up the bond requires energy because you are separating the shared electrons as they become two separate atoms. “d” has increased and so has the potential energy. We have an end ...
... closer to each other’s nucleus. The separation distance, d, is small, just like the height in the physics analogy. To break up the bond requires energy because you are separating the shared electrons as they become two separate atoms. “d” has increased and so has the potential energy. We have an end ...
Conduction electrons propagate diffusively in the system: bumping
... the same, the interference term between them do not average to zero. It turns out that the probability of finding the electron at O is twice that of the classical result because of the quantum interference. ...
... the same, the interference term between them do not average to zero. It turns out that the probability of finding the electron at O is twice that of the classical result because of the quantum interference. ...
MS PowerPoint - Catalysis Eprints database
... In terms of molecular orbital theory, since three atoms will form three molecular orbitals of different energy and these orbitals have to be filled by four electrons, two from the A-H bond and two from the lone pair of B. Out of three two orbitals will be filled by these electrons and a high energy ...
... In terms of molecular orbital theory, since three atoms will form three molecular orbitals of different energy and these orbitals have to be filled by four electrons, two from the A-H bond and two from the lone pair of B. Out of three two orbitals will be filled by these electrons and a high energy ...
Final Review Answers
... a) ammonia pyr./107°/P b) water bent/105°/P c) carbon dioxide linear/180°/NP d) carbon tetraiodide tetra/109.5°/NP 14)Differentiate between the three main types of intermolecular forces described in your text. Dipole - attraction between P molec.; Hydrogen - attraction between P molec. (H must be bo ...
... a) ammonia pyr./107°/P b) water bent/105°/P c) carbon dioxide linear/180°/NP d) carbon tetraiodide tetra/109.5°/NP 14)Differentiate between the three main types of intermolecular forces described in your text. Dipole - attraction between P molec.; Hydrogen - attraction between P molec. (H must be bo ...
1617 Ch3 Practice Test Key Student
... ii) Determine the limiting reactant. Clearly justify your answer with calculations or in words. One way to determine the limiting reactant (as explained in class) is to choose one given reactant and use it to determine how much of the other reactant is needed. Then compare this needed amount to the ...
... ii) Determine the limiting reactant. Clearly justify your answer with calculations or in words. One way to determine the limiting reactant (as explained in class) is to choose one given reactant and use it to determine how much of the other reactant is needed. Then compare this needed amount to the ...
b - PianetaChimica
... Mass spectrometry is an important tool in the determination of the structures of organic compounds. The process begins with the ionisation of the sample to form a positively charged ion, the molecular ion. At this stage, the molecular ion commonly fragments to form additional cations. These cations ...
... Mass spectrometry is an important tool in the determination of the structures of organic compounds. The process begins with the ionisation of the sample to form a positively charged ion, the molecular ion. At this stage, the molecular ion commonly fragments to form additional cations. These cations ...
rocks and minerals quiz
... raise the temperature of one gram water by 1o C. UNITS OF ENERGY A joule (J) joule is the energy needed to move a one Newton mass (about ¼ English pounds) over the distance of a meter. It is also smaller than a calorie. UNITS OF ENERGY When larger units are required, kilocalories and kilowatt-hours ...
... raise the temperature of one gram water by 1o C. UNITS OF ENERGY A joule (J) joule is the energy needed to move a one Newton mass (about ¼ English pounds) over the distance of a meter. It is also smaller than a calorie. UNITS OF ENERGY When larger units are required, kilocalories and kilowatt-hours ...
Chap-7
... enhancement. Because the third order nonlinear processes are generally weak the resonance processes are usually dominant. 2) (3) is a fourth rank tensor; so in the most general sense it contains 81 independent elements written as (3)ijkl; for the specific case of an isotropic medium there are only ...
... enhancement. Because the third order nonlinear processes are generally weak the resonance processes are usually dominant. 2) (3) is a fourth rank tensor; so in the most general sense it contains 81 independent elements written as (3)ijkl; for the specific case of an isotropic medium there are only ...
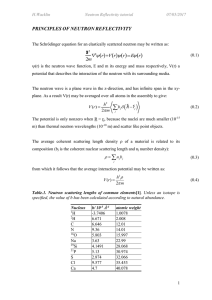
HERCULES_Neutron_reflectivity
... In the TOF mode, the neutron wavelengths are determined by recording the arrival time of each neutron at the detector relative to the start of each pulse, with shorter wavelengths arriving first and corresponding to high values of q. On steady state reactor beams, a pair of mechanical choppers is us ...
... In the TOF mode, the neutron wavelengths are determined by recording the arrival time of each neutron at the detector relative to the start of each pulse, with shorter wavelengths arriving first and corresponding to high values of q. On steady state reactor beams, a pair of mechanical choppers is us ...
Help Sheet for the Energy Calculations 2
... M(CH3OH) = 32.0 g mol–1 means that 1 mol of CH3OH has a mass of 32.0g. What happens if you are not given the M(CH3OH)? If you had to calculate from the values on the periodic table you would use the molar masses... ...
... M(CH3OH) = 32.0 g mol–1 means that 1 mol of CH3OH has a mass of 32.0g. What happens if you are not given the M(CH3OH)? If you had to calculate from the values on the periodic table you would use the molar masses... ...
rocks and minerals quiz
... raise the temperature of one gram water by 1o C. UNITS OF ENERGY A joule (J) joule is the energy needed to move a one Newton mass (about ¼ English pounds) over the distance of a meter. It is also smaller than a calorie. UNITS OF ENERGY When larger units are required, kilocalories and kilowatt-hours ...
... raise the temperature of one gram water by 1o C. UNITS OF ENERGY A joule (J) joule is the energy needed to move a one Newton mass (about ¼ English pounds) over the distance of a meter. It is also smaller than a calorie. UNITS OF ENERGY When larger units are required, kilocalories and kilowatt-hours ...
Document
... We do not really have to memorize the order of energy for all of the orbitals. Instead we use our understanding of the positions on the periodic table to copy down the correct order of energies from the periodic table. Every time the number of protons in a nucleus increases (atomic number), the num ...
... We do not really have to memorize the order of energy for all of the orbitals. Instead we use our understanding of the positions on the periodic table to copy down the correct order of energies from the periodic table. Every time the number of protons in a nucleus increases (atomic number), the num ...
chapter2
... • An example of an isotope symbol is 28 Ni. This symbol represents an isotope of nickel that contains 28 protons and 32 neutrons in the nucleus. • Isotopes are also represented by the notation: Name-A, where Name is the name of the element and A is the mass number of the isotope. • An example of thi ...
... • An example of an isotope symbol is 28 Ni. This symbol represents an isotope of nickel that contains 28 protons and 32 neutrons in the nucleus. • Isotopes are also represented by the notation: Name-A, where Name is the name of the element and A is the mass number of the isotope. • An example of thi ...
Hund`s Rule for Composite Fermions
... with the understanding that each state in this sector belongs to a degenerate multiplet of (2L + 1)(2S + 1) states. The origin of Hund’s rule in atomic physics lies in the short-range part of the Coulomb interaction. It is most clearly explained by modeling the Coulomb interaction by a repulsive del ...
... with the understanding that each state in this sector belongs to a degenerate multiplet of (2L + 1)(2S + 1) states. The origin of Hund’s rule in atomic physics lies in the short-range part of the Coulomb interaction. It is most clearly explained by modeling the Coulomb interaction by a repulsive del ...
Non-thermal laser-induced desorption of metal atoms with bimodal
... of a Nd : YAG laser at j"1064, 532, 355 and 266 nm in order to stimulate desorption of atoms from the surface of the Na particles. The angle of incidence was 50° with respect to the surface normal and the pulse duration about 7 ns at a repetition rate of 10 Hz. Laser fluences ranging from 0.1 up to ...
... of a Nd : YAG laser at j"1064, 532, 355 and 266 nm in order to stimulate desorption of atoms from the surface of the Na particles. The angle of incidence was 50° with respect to the surface normal and the pulse duration about 7 ns at a repetition rate of 10 Hz. Laser fluences ranging from 0.1 up to ...
1 of 8 PH1503 - PROPERTIES OF MATTER AND ACOUSTICS
... Semester : I No. of hrs/wk : 4 Objective: This paper is offered to the students of mathematics as allied required. Most of the mathematics learnt by the students has immediate application to many physical problems. The logical reasoning behind the description of the physics problem and obtaining the ...
... Semester : I No. of hrs/wk : 4 Objective: This paper is offered to the students of mathematics as allied required. Most of the mathematics learnt by the students has immediate application to many physical problems. The logical reasoning behind the description of the physics problem and obtaining the ...
Interferometric back focal plane microellipsometry
... thick aluminum film evaporated onto a glass microscope slide, and a thick gold film evaporated onto a silicon wafer. Resultant difference maps are shown in Fig. 5. In the Au and Al data, d increases and decreases along the horizontal and vertical directions, respectively, as expected from Eqs. ~17!, ...
... thick aluminum film evaporated onto a glass microscope slide, and a thick gold film evaporated onto a silicon wafer. Resultant difference maps are shown in Fig. 5. In the Au and Al data, d increases and decreases along the horizontal and vertical directions, respectively, as expected from Eqs. ~17!, ...
Spontaneous Emission Spectrum in Double Quantum Dot Devices
... absorption with stimulated and spontaneous emission. The most effectively coupled bosons in the specific environment of the semiconductor device used here were acoustic phonons. The experiments demonstrate the importance of vacuum fluctuations in the environment for quantum dot devices and potential ...
... absorption with stimulated and spontaneous emission. The most effectively coupled bosons in the specific environment of the semiconductor device used here were acoustic phonons. The experiments demonstrate the importance of vacuum fluctuations in the environment for quantum dot devices and potential ...
Lab
... beam refracted by the prism has an angle of minimum deviation. It means that the incident angle is equal to the refraction angle, i = r, when the laser beam has a minimum deviation through the prism. (3) How large error are the both measured apex angles and the angle of minimum obtained in thi ...
... beam refracted by the prism has an angle of minimum deviation. It means that the incident angle is equal to the refraction angle, i = r, when the laser beam has a minimum deviation through the prism. (3) How large error are the both measured apex angles and the angle of minimum obtained in thi ...
Fermion-Fermion and Boson-Boson Interactions at low
... Both Bosons and Fermions show quantum effects when cooled down below Bosons macroscopically occupy the ground state → Bose-Einstein-Condensation Fermions form Cooper-Pairs and exhibit superfluid behavior Interactions at low energy are characterized by the scattering length a Variation of a ...
... Both Bosons and Fermions show quantum effects when cooled down below Bosons macroscopically occupy the ground state → Bose-Einstein-Condensation Fermions form Cooper-Pairs and exhibit superfluid behavior Interactions at low energy are characterized by the scattering length a Variation of a ...
as a PDF
... In the latter type of reaction, the second part of our principle states that the energy variation is nearly smooth. Why do we need the qualification “nearly”? First, there are many important chemical changes to which thermodynamics is rather insensitive. Structure is often a good example. The smooth ...
... In the latter type of reaction, the second part of our principle states that the energy variation is nearly smooth. Why do we need the qualification “nearly”? First, there are many important chemical changes to which thermodynamics is rather insensitive. Structure is often a good example. The smooth ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.