Atomic Theory - WaylandHighSchoolChemistry
... Song about Atomic Theory Notes on Atomic Theory ...
... Song about Atomic Theory Notes on Atomic Theory ...
C 3 HAPTER
... Fourier transform infrared (FTIR) spectroscopy is used to measure the vibration of a molecule which is a unique physical property of a molecule (Coates, 2000). IR occurs between the visible and microwave radiation. IR waves spans from the near-IR (13 000-4000 cm-1); mid-IR (4000-400 cm-1) and far-IR ...
... Fourier transform infrared (FTIR) spectroscopy is used to measure the vibration of a molecule which is a unique physical property of a molecule (Coates, 2000). IR occurs between the visible and microwave radiation. IR waves spans from the near-IR (13 000-4000 cm-1); mid-IR (4000-400 cm-1) and far-IR ...
This `practice exam`
... NOTE: This ‘practice exam’ contains more than questions than the real final. 1. The wavelength of light emitted from a green laser pointer is 5.32 × 102 nm. What is the wavelength in meters? 5.32 × 10-7 m 2. What is the correct answer, with correct significant figures, to the following expression: ( ...
... NOTE: This ‘practice exam’ contains more than questions than the real final. 1. The wavelength of light emitted from a green laser pointer is 5.32 × 102 nm. What is the wavelength in meters? 5.32 × 10-7 m 2. What is the correct answer, with correct significant figures, to the following expression: ( ...
chapter 7 – cyu
... shells outside of the nucleus of an atom. 2. A gas discharge tube is a device originally designed by Davy and refined by Geissler to remove the particles of air within the tube, therefore only allowing the electrons to show up. 3. Crookes put an iron cross in the middle. The cross blocked the rays c ...
... shells outside of the nucleus of an atom. 2. A gas discharge tube is a device originally designed by Davy and refined by Geissler to remove the particles of air within the tube, therefore only allowing the electrons to show up. 3. Crookes put an iron cross in the middle. The cross blocked the rays c ...
1 - shawnschmitt
... g. Mole- the amount of particles in 12g of Carbon-12, also, the amount of substance having 6.022x1023 of any kind of particle h. half-life- the amount of time required for ½ of the mass of an isotope to decay i. metalloid- those elements that have properties of both metals and nonmetals j. Ionizatio ...
... g. Mole- the amount of particles in 12g of Carbon-12, also, the amount of substance having 6.022x1023 of any kind of particle h. half-life- the amount of time required for ½ of the mass of an isotope to decay i. metalloid- those elements that have properties of both metals and nonmetals j. Ionizatio ...
Atomic Structure: SOL Review #1 Name: Historical Developments 1
... all things made of tiny particles solid sphere model; Atomic Theory: all elements made of atoms, atoms of an element are identical cathode ray tube; discovered electron; plum pudding model oil drop; discovered mass and charge of electron gold foil; discovered nucleus; the atom is mostly empty space ...
... all things made of tiny particles solid sphere model; Atomic Theory: all elements made of atoms, atoms of an element are identical cathode ray tube; discovered electron; plum pudding model oil drop; discovered mass and charge of electron gold foil; discovered nucleus; the atom is mostly empty space ...
FRAUNHOFER and FRESNEL DIFFRACTION
... • Diffraction aperture plate with various circular apertures • Optical rail and riders ...
... • Diffraction aperture plate with various circular apertures • Optical rail and riders ...
08_lecture_ppt - Chemistry at Winthrop University
... • Bohr theory only modeled the line spectrum of H • Further experiments established waveparticle duality of light and matter – Young’s two slit experiment produced interference patterns for both photons and electrons. ...
... • Bohr theory only modeled the line spectrum of H • Further experiments established waveparticle duality of light and matter – Young’s two slit experiment produced interference patterns for both photons and electrons. ...
Lecture 12: Fraunhofer diffraction by a single slit
... electric field of all wavelets from all original segments taking into account difference in optical path length and amplitude Why do we study diffraction on slits, circular apertures etc: to understand basics, and due to high relevance to applications ...
... electric field of all wavelets from all original segments taking into account difference in optical path length and amplitude Why do we study diffraction on slits, circular apertures etc: to understand basics, and due to high relevance to applications ...
Chapter 3 Crystallography and Diffraction Techniques
... accelerated towards an anode (attached with a piece of Cu) by a voltage of ~ 30 kV. The chamber is known as the X-ray tube, is evacuated to prevent W oxidation. Be windows are very suitable for X-ray passing through, because Be has an atomic number of 4 (non-absorbing). Lead is very effective in shi ...
... accelerated towards an anode (attached with a piece of Cu) by a voltage of ~ 30 kV. The chamber is known as the X-ray tube, is evacuated to prevent W oxidation. Be windows are very suitable for X-ray passing through, because Be has an atomic number of 4 (non-absorbing). Lead is very effective in shi ...
Theory & Implementation of the Scanning Tunneling Microscope
... If two metals are connected to opposite ends of a battery but are separated from each other than they logically should not conduct electricity. However, as we bring the metals extremely close to each other the wavefunctions of the electrons are able to tunnel through the gap and we will detect a cur ...
... If two metals are connected to opposite ends of a battery but are separated from each other than they logically should not conduct electricity. However, as we bring the metals extremely close to each other the wavefunctions of the electrons are able to tunnel through the gap and we will detect a cur ...
Document
... It is important for you to come to class prepared, i.e. be familiar with the material to be presented. To test your preparedness, a simple five-minute quiz, testing your qualitative familiarity with the material to be discussed in class, will be given at the beginning of some of the classes. No make ...
... It is important for you to come to class prepared, i.e. be familiar with the material to be presented. To test your preparedness, a simple five-minute quiz, testing your qualitative familiarity with the material to be discussed in class, will be given at the beginning of some of the classes. No make ...
Semester Exam Review Guide
... 26. If the mass of a steel bolt is 4.0 grams and its volume is 2 milliliters, what is the bolt’s density? a. 2 ml / g b. 2 g / ml c. .5 g / ml d. 8 ml / g 27. How many Hydrogen atoms are in the following chemical unit: 5HN2(OH)2 a. 10 b. 5 c. 15 d. 7 ...
... 26. If the mass of a steel bolt is 4.0 grams and its volume is 2 milliliters, what is the bolt’s density? a. 2 ml / g b. 2 g / ml c. .5 g / ml d. 8 ml / g 27. How many Hydrogen atoms are in the following chemical unit: 5HN2(OH)2 a. 10 b. 5 c. 15 d. 7 ...
Surface Plasmon Resonance
... evanescent wave: - nearfield standing wave, - extends about 1/2 , - decays exponentially with the distance ...
... evanescent wave: - nearfield standing wave, - extends about 1/2 , - decays exponentially with the distance ...
which technique or techniques would be most appropriate for use in
... X-Rays are detected by the ionization that they cause. In vacuum tube designs the X-rays ionize a low pressure gas and these ions are detected. The X-ray energy can be analyzed with an X-ray monochrometer that uses a salt crystal in place of a grating. In solid state X-ray detectors, the X-rays crea ...
... X-Rays are detected by the ionization that they cause. In vacuum tube designs the X-rays ionize a low pressure gas and these ions are detected. The X-ray energy can be analyzed with an X-ray monochrometer that uses a salt crystal in place of a grating. In solid state X-ray detectors, the X-rays crea ...
Solid-state physics
... Many properties of materials are affected by their crystal structure. This structure can be investigated using a range of crystallographic techniques, including X-ray crystallography, neutron diffraction and electron diffraction. The sizes of the individual crystals in a crystalline solid material v ...
... Many properties of materials are affected by their crystal structure. This structure can be investigated using a range of crystallographic techniques, including X-ray crystallography, neutron diffraction and electron diffraction. The sizes of the individual crystals in a crystalline solid material v ...
Chapter 7 Ionic and Metallic Bonding 1. What are valence electrons
... 5. Draw the electron dot structure for the following: Ar, Ca, and I. ...
... 5. Draw the electron dot structure for the following: Ar, Ca, and I. ...
Electron
... Resolution limitations of the VLM • 1839, George Airy: there should be a natural limit to the optical microscopes. • 1872, both Ernst Abbe and Hermann von Helmholtz: Light is limited by the size of the wavelength. Resolution of the eyes 0.1-0.2 mm Resolution of a good VLM ~300 nm ...
... Resolution limitations of the VLM • 1839, George Airy: there should be a natural limit to the optical microscopes. • 1872, both Ernst Abbe and Hermann von Helmholtz: Light is limited by the size of the wavelength. Resolution of the eyes 0.1-0.2 mm Resolution of a good VLM ~300 nm ...
MLSystems Lab 1 - Fourier v4 - RIT
... These discrete coefficients are the diffraction orders of the Fraunhofer diffraction pattern that are produced when a diffraction grating is illuminated by coherent illumination. These coefficients, represented as terms in the harmonic decomposition of m(x) correspond to the discrete orders seen in ...
... These discrete coefficients are the diffraction orders of the Fraunhofer diffraction pattern that are produced when a diffraction grating is illuminated by coherent illumination. These coefficients, represented as terms in the harmonic decomposition of m(x) correspond to the discrete orders seen in ...
For this basic module we simply take the suitable module
... swarm about with constant drift velocity. Basic mechanics yields for a single particle with momentum p F = dp/dt = m·dv/dt with p = momentum of the electron. Note that p does not have to be zero when the field is switched on. If this would be all, the velocity of a given electron would acquire an ev ...
... swarm about with constant drift velocity. Basic mechanics yields for a single particle with momentum p F = dp/dt = m·dv/dt with p = momentum of the electron. Note that p does not have to be zero when the field is switched on. If this would be all, the velocity of a given electron would acquire an ev ...
Chapter 5 - Blair Community Schools
... Atoms and ions Different chemical properties. Metals --> cations, less energy to lose electrons (1 to 3) than to gain (5 to 7) Nonmetals --> anions, less energy to gain electrons (1 to 3) than lose (5 to 7) Cations and anions combine to form compounds with very specific properties separate fr ...
... Atoms and ions Different chemical properties. Metals --> cations, less energy to lose electrons (1 to 3) than to gain (5 to 7) Nonmetals --> anions, less energy to gain electrons (1 to 3) than lose (5 to 7) Cations and anions combine to form compounds with very specific properties separate fr ...
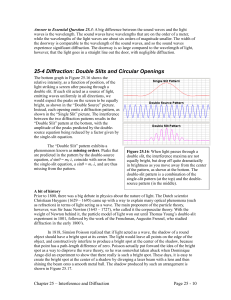
25-4 Diffraction: Double Slits and Circular Openings
... all look through, the pupil in each of our own eyes. For a circular opening, the angle at which the first zero occurs in a diffraction pattern is given by: (Eq. 25.7: The first zero in a diffraction pattern from a circular aperture) where D is the diameter of the opening. Note that the larger the di ...
... all look through, the pupil in each of our own eyes. For a circular opening, the angle at which the first zero occurs in a diffraction pattern is given by: (Eq. 25.7: The first zero in a diffraction pattern from a circular aperture) where D is the diameter of the opening. Note that the larger the di ...
Low-energy electron diffraction

Low-energy electron diffraction (LEED) is a technique for the determination of the surface structure of single-crystalline materials by bombardment with a collimated beam of low energy electrons (20–200 eV) and observation of diffracted electrons as spots on a fluorescent screen.LEED may be used in one of two ways: Qualitatively, where the diffraction pattern is recorded and analysis of the spot positions gives information on the symmetry of the surface structure. In the presence of an adsorbate the qualitative analysis may reveal information about the size and rotational alignment of the adsorbate unit cell with respect to the substrate unit cell. Quantitatively, where the intensities of diffracted beams are recorded as a function of incident electron beam energy to generate the so-called I-V curves. By comparison with theoretical curves, these may provide accurate information on atomic positions on the surface at hand.↑