Study Guide
... Predict the oxidation states of elements in ionic compounds. What is the charge of a magnesium ion? What is the charge of a lithium ion? What is the charge of a sulfide ion? What is the charge of a bromide ion? ...
... Predict the oxidation states of elements in ionic compounds. What is the charge of a magnesium ion? What is the charge of a lithium ion? What is the charge of a sulfide ion? What is the charge of a bromide ion? ...
HONG KONG DIPLOMA OF SECONDARY EDUCATION
... Mars is the fourth planet from the Sun. Mars has a very different atmosphere from that of the Earth. The atmosphere is relatively thin with an average pressure of 0.6 kilopascals (kPa) (compared to Earth’s 101.3 kPa). It is actually less than 1% the atmospheric density of Earth. Therefore, there is ...
... Mars is the fourth planet from the Sun. Mars has a very different atmosphere from that of the Earth. The atmosphere is relatively thin with an average pressure of 0.6 kilopascals (kPa) (compared to Earth’s 101.3 kPa). It is actually less than 1% the atmospheric density of Earth. Therefore, there is ...
Equilibrium Constant- Keq
... a) Write the equilibrium equation and equilibrium law for this reaction. b) The equilibrium concentrations in this system are [HBr(g)] =0.240 mol/L and [H2(g)]=0.130 mol/L and [Br2(g)] = 0.130 mol/L. Calculate Keq. 2. Nitrogen dioxide gas (4.6 mol/L) is produced from nitrogen monoxide gas (1.3 mol/L ...
... a) Write the equilibrium equation and equilibrium law for this reaction. b) The equilibrium concentrations in this system are [HBr(g)] =0.240 mol/L and [H2(g)]=0.130 mol/L and [Br2(g)] = 0.130 mol/L. Calculate Keq. 2. Nitrogen dioxide gas (4.6 mol/L) is produced from nitrogen monoxide gas (1.3 mol/L ...
Writing Chemical Formulas and Chemical Reactions
... All chemical equations must be balanced so that they are consistent with the Law of Conservation of Mass. Here are some suggestions for balancing equations: 1. When balancing equations, always start with the “ugliest” molecule first (polyatomics). 2. To balance, place the desired number (coefficient ...
... All chemical equations must be balanced so that they are consistent with the Law of Conservation of Mass. Here are some suggestions for balancing equations: 1. When balancing equations, always start with the “ugliest” molecule first (polyatomics). 2. To balance, place the desired number (coefficient ...
Period - scienceathylands
... • The number of protons increases, so there is more attraction acting on the electrons • Electrons are added to the same shell, so the outer shell is drawn inwards slightly. Same no. of shells = same level of shielding, but more nuclear charge. ...
... • The number of protons increases, so there is more attraction acting on the electrons • Electrons are added to the same shell, so the outer shell is drawn inwards slightly. Same no. of shells = same level of shielding, but more nuclear charge. ...
AP Chemistry
... (with either a catalyst or heat) 2 H2O2(g) → 2 H2O(g) + O2(g) c) Chlorates 2KClO3(s) Δ → 2KCl(s) + 3O2(g) d) Carbonates (NH4)2CO3(s) Δ → H2O(g) + CO2(g) + 2NH3(g) CaCO3(s) Δ → CaO(s) + CO2(g) NaHCO3(s) Δ → Na2CO3(s) + H2O(g) + CO2(g) C) Single Displacement (Redox) An element reacts with a compound t ...
... (with either a catalyst or heat) 2 H2O2(g) → 2 H2O(g) + O2(g) c) Chlorates 2KClO3(s) Δ → 2KCl(s) + 3O2(g) d) Carbonates (NH4)2CO3(s) Δ → H2O(g) + CO2(g) + 2NH3(g) CaCO3(s) Δ → CaO(s) + CO2(g) NaHCO3(s) Δ → Na2CO3(s) + H2O(g) + CO2(g) C) Single Displacement (Redox) An element reacts with a compound t ...
The Periodic Table
... 13. A period (a row) on the periodic table has elements that have what in common… A. They have similar chemical properties B. They have the same number of electrons C. They have the same number of electron shells D. They are similarly reactive in compounds. 14. The vertical columns in a periodic tab ...
... 13. A period (a row) on the periodic table has elements that have what in common… A. They have similar chemical properties B. They have the same number of electrons C. They have the same number of electron shells D. They are similarly reactive in compounds. 14. The vertical columns in a periodic tab ...
The Periodic Table - Science Education at Jefferson Lab
... share electrons when forming compounds. • Oxygen is the most abundant element in the earth’s crust. It is extremely active and combines with almost all elements. ...
... share electrons when forming compounds. • Oxygen is the most abundant element in the earth’s crust. It is extremely active and combines with almost all elements. ...
ASFG High School Summer Assignment Summer 2016
... f. Krypton g. Fluorine h. Scandium I. Arsenic J. Potassium K. Sodium l. chloride m. Iron n. Zinc ...
... f. Krypton g. Fluorine h. Scandium I. Arsenic J. Potassium K. Sodium l. chloride m. Iron n. Zinc ...
R The Periodic Table
... •not able to conduct electricity or heat very well •exist in two of the three states of matter at room temperature: gases (such as oxygen) and solids (such as carbon). •very brittle, and cannot be rolled into wires or pounded into sheets •have no metallic luster, and do not reflect light. ...
... •not able to conduct electricity or heat very well •exist in two of the three states of matter at room temperature: gases (such as oxygen) and solids (such as carbon). •very brittle, and cannot be rolled into wires or pounded into sheets •have no metallic luster, and do not reflect light. ...
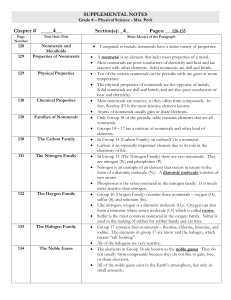
Families of Elements
... Elements in group IA of the periodic table, with the exception of hydrogen Have one electron in their outer energy levels Are the most chemically active of all metals (meaning an element readily combines with other substances to form compounds) NEVER found in pure form A way to identify al ...
... Elements in group IA of the periodic table, with the exception of hydrogen Have one electron in their outer energy levels Are the most chemically active of all metals (meaning an element readily combines with other substances to form compounds) NEVER found in pure form A way to identify al ...
Bonding Web Practice Trupia - Trupia
... ____3. Which symbol represents a particle that has the same total number of electrons as S2–? (3) Se2– (1) O2– (2) Si (4) Ar ____4. Which element has atoms with the greatest attraction for electrons in a chemical bond? (1) beryllium (3) lithium (2) fluorine (4) oxygen ____5. Which atom will form the ...
... ____3. Which symbol represents a particle that has the same total number of electrons as S2–? (3) Se2– (1) O2– (2) Si (4) Ar ____4. Which element has atoms with the greatest attraction for electrons in a chemical bond? (1) beryllium (3) lithium (2) fluorine (4) oxygen ____5. Which atom will form the ...
Name - TeacherWeb
... Group 17 contains four nonmetals – fluorine, chlorine, bromine, and iodine. The elements in group 17 are know and the halogen, which means “salt forming”. All of the halogens are very reactive. The elements in Group 18 are known as the noble gases. They do not usually form compounds because they do ...
... Group 17 contains four nonmetals – fluorine, chlorine, bromine, and iodine. The elements in group 17 are know and the halogen, which means “salt forming”. All of the halogens are very reactive. The elements in Group 18 are known as the noble gases. They do not usually form compounds because they do ...
4.3 Exploring the Modern Periodic Table
... bonds with the following elements: (hint: compare the column # below with the example above) (silicon is in column # ...
... bonds with the following elements: (hint: compare the column # below with the example above) (silicon is in column # ...
Problem Set: Empirical and Molecular Formulas
... agent for paper. It can be made by reacting O2 with TiCl4. TiCl4 + O2 TiO2 + 2 Cl2 (already balanced) a) If 4.5 mol of TiCl4 react with 3.5 mol O2, identify both the limiting and excess reactants. b) How many moles of excess reactant will remain if the reaction goes to ...
... agent for paper. It can be made by reacting O2 with TiCl4. TiCl4 + O2 TiO2 + 2 Cl2 (already balanced) a) If 4.5 mol of TiCl4 react with 3.5 mol O2, identify both the limiting and excess reactants. b) How many moles of excess reactant will remain if the reaction goes to ...
Example
... ductile (pulled into a wire), and malleable (pounded into flat sheets) • Transition metals: Metals that can have two different valence numbers. Is shown in the formula. Example: Fe (II) ...
... ductile (pulled into a wire), and malleable (pounded into flat sheets) • Transition metals: Metals that can have two different valence numbers. Is shown in the formula. Example: Fe (II) ...
Structure of the Atom and Periodic Table Quiz 2016 Self
... The vertical columns, called groups describe elements of similar properties. Groups have names that help describe the properties those elements share. An example if the reactive metals on the far left because they are all very reactive. As you move across a period, the properties change in predictab ...
... The vertical columns, called groups describe elements of similar properties. Groups have names that help describe the properties those elements share. An example if the reactive metals on the far left because they are all very reactive. As you move across a period, the properties change in predictab ...
Day 72 TYPES OF CHEMICAL REACTIONS
... Release of energy as heat Release of energy as light Change in colour ...
... Release of energy as heat Release of energy as light Change in colour ...
Periodic Table and Trends
... The top, right-area of the periodic table has the smallest atomic radius (helium) Which would you assume to have the largest atomic radius: Al, Al+, or AlWhich would you assume to have the smallest atomic radius: C2+, C+, C, C-, C2- ...
... The top, right-area of the periodic table has the smallest atomic radius (helium) Which would you assume to have the largest atomic radius: Al, Al+, or AlWhich would you assume to have the smallest atomic radius: C2+, C+, C, C-, C2- ...
2 - DrChoChemistryWebSite
... 3) Balance the elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the H and O until LAST! ...
... 3) Balance the elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the H and O until LAST! ...
The Periodic Table
... periodic table and determine how they will combine with elements of different ...
... periodic table and determine how they will combine with elements of different ...
Chemistry Study Cards Chapter 5 (3-2) The length of each period in
... gained, or shared when atoms form molecules are called When chemical compounds form, lost, gained, or shared. valence electrons are those that may be The number of valence electrons in Group 1 elements is ...
... gained, or shared when atoms form molecules are called When chemical compounds form, lost, gained, or shared. valence electrons are those that may be The number of valence electrons in Group 1 elements is ...
Chapter 5 Section 1 - Ms. Halbohm`s Classroom
... c. First ionization energy d. Second ionization energy 3. How do first ionization energies of main-group elements vary across a period and down a group? Explain the basis for each trend. 4. What is electron affinity? What signs are associated with electron affinity values, and what is the significan ...
... c. First ionization energy d. Second ionization energy 3. How do first ionization energies of main-group elements vary across a period and down a group? Explain the basis for each trend. 4. What is electron affinity? What signs are associated with electron affinity values, and what is the significan ...