Recombinant Human Myosin VIIa protein ab152555 Product datasheet 1 Image Overview
... Our Abpromise to you: Quality guaranteed and expert technical support Replacement or refund for products not performing as stated on the datasheet Valid for 12 months from date of delivery Response to your inquiry within 24 hours We provide support in Chinese, English, French, German, Japanese and S ...
... Our Abpromise to you: Quality guaranteed and expert technical support Replacement or refund for products not performing as stated on the datasheet Valid for 12 months from date of delivery Response to your inquiry within 24 hours We provide support in Chinese, English, French, German, Japanese and S ...
Lecture 13-Effects of glycosylation on protein structure and function
... are exposed to solvent • Residues involved in H-‐bonding between strands of the β-‐sheet show low rates of exchange with deuterated water • At rt, the exchange rates for all of these amide protons ...
... are exposed to solvent • Residues involved in H-‐bonding between strands of the β-‐sheet show low rates of exchange with deuterated water • At rt, the exchange rates for all of these amide protons ...
DNA and RNA: Composition and Structure
... • Denaturation or inhibition may change protein structure - will change its function • Coenzyme and co factor may enhance the protein’s structure ...
... • Denaturation or inhibition may change protein structure - will change its function • Coenzyme and co factor may enhance the protein’s structure ...
Link to DOC - VCU Department of Physiology and Biophysics
... outer leaflet of the inner membrane (IM), is believed to be mediated by the O-Ag flippase Wzx, an integral IM protein. While Wzx proteins are found in a wide range of bacteria, structural data to explain their purported function was non-existent until a recent investigation by our group in which the ...
... outer leaflet of the inner membrane (IM), is believed to be mediated by the O-Ag flippase Wzx, an integral IM protein. While Wzx proteins are found in a wide range of bacteria, structural data to explain their purported function was non-existent until a recent investigation by our group in which the ...
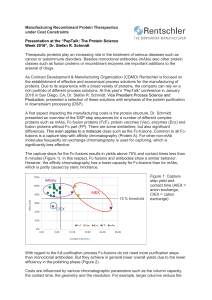
Manufacturing Recombinant Protein Therapeutics under Cost
... in downstream processing (DSP). A first aspect impacting the manufacturing costs is the protein structure. Dr. Schmidt presented an overview of the DSP step sequences for a number of different complex proteins such as mAbs, Fc-fusion proteins (FcF), protein vaccines (Vac), enzymes (Enz) and fusion p ...
... in downstream processing (DSP). A first aspect impacting the manufacturing costs is the protein structure. Dr. Schmidt presented an overview of the DSP step sequences for a number of different complex proteins such as mAbs, Fc-fusion proteins (FcF), protein vaccines (Vac), enzymes (Enz) and fusion p ...
Protein
... grains, some vegetables, and some fruits (provide only small amounts of protein relative to other sources) As we mentioned, most adults in the United States get more than enough protein to meet their needs. It's rare for someone who is healthy and eating a varied diet to not get enough protein. Wha ...
... grains, some vegetables, and some fruits (provide only small amounts of protein relative to other sources) As we mentioned, most adults in the United States get more than enough protein to meet their needs. It's rare for someone who is healthy and eating a varied diet to not get enough protein. Wha ...
supersecondar, tertiary and quaternary structure
... side groups must be buried inside the folds, therefore, layers must be created (b-a-b; a-a). ...
... side groups must be buried inside the folds, therefore, layers must be created (b-a-b; a-a). ...
The in vitro catalysis of protein folding by endoplasmic reticulum
... Protein folding in the cell is assisted by niany auxiliary proteins that catalyse covalent isomerisation steps, or ‘chaperone’ the folding of nascent chains and prevent them from entering non-productive pathways (1.2). Several catalysts and chaperones have now been identified which assist in such ce ...
... Protein folding in the cell is assisted by niany auxiliary proteins that catalyse covalent isomerisation steps, or ‘chaperone’ the folding of nascent chains and prevent them from entering non-productive pathways (1.2). Several catalysts and chaperones have now been identified which assist in such ce ...
BIOGRAPHICAL SKETCH Abhijeet Kapoor Postdoctoral Research
... 2015Postdoctoral Research Associate, Department of Chemical and Structural Biology, Icahn School of Medicine at Mount Sinai, USA Other Experience, Professional Memberships, and Awards ...
... 2015Postdoctoral Research Associate, Department of Chemical and Structural Biology, Icahn School of Medicine at Mount Sinai, USA Other Experience, Professional Memberships, and Awards ...
Multipower Sportsfood launches Fit Protein Lite
... Fit Protein Lite delivers 80% less carbs and sugars than Multipower’s number one selling Fit Protein in the iconic brown bottle. Retailing at just £3.85 a bottle the 500ml drink is available in three delicious flavours of Chocolate, Vanilla and Strawberry. Multipower Nutritionist Drew Price said: “F ...
... Fit Protein Lite delivers 80% less carbs and sugars than Multipower’s number one selling Fit Protein in the iconic brown bottle. Retailing at just £3.85 a bottle the 500ml drink is available in three delicious flavours of Chocolate, Vanilla and Strawberry. Multipower Nutritionist Drew Price said: “F ...
USMLE Step 1 Web Prep — The Genetic Code, Mutations, and
... 1. Primary--sequence of amino acids specified in the gene. 2. Secondary--folding of the amino acid chain into an energetically stable structure, either into an alpha-helix, or a beta-pleated-sheet. 3. Tertiary--positioning of the secondary structures in relation to each other to generate higher-orde ...
... 1. Primary--sequence of amino acids specified in the gene. 2. Secondary--folding of the amino acid chain into an energetically stable structure, either into an alpha-helix, or a beta-pleated-sheet. 3. Tertiary--positioning of the secondary structures in relation to each other to generate higher-orde ...
western blot - IISME Community Site
... of a solution. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. Many life forms thrive only in a relatively small pH range so they utilize a buffer solution to maintain a constant pH. ...
... of a solution. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. Many life forms thrive only in a relatively small pH range so they utilize a buffer solution to maintain a constant pH. ...
Alzheimer`s - Science Nutshell
... be obtained from alternatively spliced mRNA. These mutations lead to an amyloidogenic form of beta amyloid peptide (A42/43), which is longer in length. The most severe mutations cause large a decrease in A40, and a large increase in A42. A40 doesn’t bind to more than 3 other A40 proteins, where ...
... be obtained from alternatively spliced mRNA. These mutations lead to an amyloidogenic form of beta amyloid peptide (A42/43), which is longer in length. The most severe mutations cause large a decrease in A40, and a large increase in A42. A40 doesn’t bind to more than 3 other A40 proteins, where ...
Donwload Nomination Form - Protein Society of Thailand
... Institutional Affiliation and Current Position: ...
... Institutional Affiliation and Current Position: ...
TERTIARY STRUCTURE OF PROTEINS
... Several important principles: • Secondary structures form wherever possible (due to formation of large numbers of H bonds) • Helices and sheets often pack close together • Peptide segments between secondary structures tend to be short and direct • Proteins fold so as to form the most stable structur ...
... Several important principles: • Secondary structures form wherever possible (due to formation of large numbers of H bonds) • Helices and sheets often pack close together • Peptide segments between secondary structures tend to be short and direct • Proteins fold so as to form the most stable structur ...
The Power of Protein - Jackson County Sheriff
... When we think protein, we think beef or pork. They have about 15-20 grams in a 3-ounce serving (the size of a deck of cards). But beef and pork can have 10+ grams of artery-clogging saturated fat in a 3-ounce serving, too. ...
... When we think protein, we think beef or pork. They have about 15-20 grams in a 3-ounce serving (the size of a deck of cards). But beef and pork can have 10+ grams of artery-clogging saturated fat in a 3-ounce serving, too. ...
LectureIV
... X-ray crystallography and NMR are the two major techniques for determining protein structures ...
... X-ray crystallography and NMR are the two major techniques for determining protein structures ...
TWO GENES ENCODING FUNCTIONAL PECTIN
... A proteinaceous inhibitor of pectin methylesterase (PMEI) has been reported in kiwi but to date no other proteins acting as PMEI have been found in plants. Two sequences closely related to PMEI from kiwi were identified in Arabidopsis thaliana. The corresponding cDNAs encode cell wall proteins of 17 ...
... A proteinaceous inhibitor of pectin methylesterase (PMEI) has been reported in kiwi but to date no other proteins acting as PMEI have been found in plants. Two sequences closely related to PMEI from kiwi were identified in Arabidopsis thaliana. The corresponding cDNAs encode cell wall proteins of 17 ...
Carbohydrates
... - Shape = globular protein - Bonds = H-bonds, Van der Waals forces, ionic bonds, hydrophobic interactions, and disulfide bridges (covalent) bonds stabilize protein conformation. Interactions between R-Groups o H-bonds between polar R groups. o Hydrophobic interactions (due to presence of water) betw ...
... - Shape = globular protein - Bonds = H-bonds, Van der Waals forces, ionic bonds, hydrophobic interactions, and disulfide bridges (covalent) bonds stabilize protein conformation. Interactions between R-Groups o H-bonds between polar R groups. o Hydrophobic interactions (due to presence of water) betw ...
Protein Structure Determination and Design
... Part II: Model Design Practice. 1. Open one of your selected PDB file structures in Jmol. 2. Change the background color to white. 3. Display and color the alpha carbon backbone of your protein model. 4. Highlight the secondary structures in your protein model. 5. Practice saving your model as a JP ...
... Part II: Model Design Practice. 1. Open one of your selected PDB file structures in Jmol. 2. Change the background color to white. 3. Display and color the alpha carbon backbone of your protein model. 4. Highlight the secondary structures in your protein model. 5. Practice saving your model as a JP ...
chapter3_Sections 4
... linear sequence of amino acids (a polypeptide chain). Each type of protein has a unique primary structure. ...
... linear sequence of amino acids (a polypeptide chain). Each type of protein has a unique primary structure. ...
Protein folding

Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil.Each protein exists as an unfolded polypeptide or random coil when translated from a sequence of mRNA to a linear chain of amino acids. This polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), known as the native state. The resulting three-dimensional structure is determined by the amino acid sequence (Anfinsen's dogma). Experiments beginning in the 1980s indicate the codon for an amino acid can also influence protein structure.The correct three-dimensional structure is essential to function, although some parts of functional proteins may remain unfolded, so that protein dynamics is important. Failure to fold into native structure generally produces inactive proteins, but in some instances misfolded proteins have modified or toxic functionality. Several neurodegenerative and other diseases are believed to result from the accumulation of amyloid fibrils formed by misfolded proteins. Many allergies are caused by incorrect folding of some proteins, because the immune system does not produce antibodies for certain protein structures.