Slide 1
... •Free amino group (-NH2) •Free carboxyl group (-COOH) •Both groups linked to a central carbon (C) ...
... •Free amino group (-NH2) •Free carboxyl group (-COOH) •Both groups linked to a central carbon (C) ...
Biochem Review, Part I: Protein Structure and Function
... • Tertiary structure can be complex and does not typically consist of repeating units. • A polypeptide will adopt the most stable tertiary structure In aqueous environment, this occurs when hydropobic residues are internal and hydrophilic residues are external. ...
... • Tertiary structure can be complex and does not typically consist of repeating units. • A polypeptide will adopt the most stable tertiary structure In aqueous environment, this occurs when hydropobic residues are internal and hydrophilic residues are external. ...
alborz-final
... antibody that is present on the array surface as in NAPPA. If any step of this process does not take place successfully then the translated protein will not bind to the surface antibody because the terminal peptide is the last segment to be translated. Hence it is possible to produce highly specific ...
... antibody that is present on the array surface as in NAPPA. If any step of this process does not take place successfully then the translated protein will not bind to the surface antibody because the terminal peptide is the last segment to be translated. Hence it is possible to produce highly specific ...
Purification of GST::TaABF1 Fusion Protein in Order to Assess its
... We are able to successfully purify GST::TaABF1 fusion protein from bacteria cultures GST::TaABF1 fusion protein was successfully recovered after endosperm phosphorylation assay Phosphorylation was not detected on the GST::TaABF1 protein using mass spectrometry ...
... We are able to successfully purify GST::TaABF1 fusion protein from bacteria cultures GST::TaABF1 fusion protein was successfully recovered after endosperm phosphorylation assay Phosphorylation was not detected on the GST::TaABF1 protein using mass spectrometry ...
Techniques of Protein and Nucleic Acid Purification
... SDS is a detergent that denatures proteins and binds strongly to proteins Most proteins bind SDS at a constant ratio (~ 1 SDS molecule per 2 residues) Swamps native charge of protein Results in average constant charge density AND similar shape for all proteins ...
... SDS is a detergent that denatures proteins and binds strongly to proteins Most proteins bind SDS at a constant ratio (~ 1 SDS molecule per 2 residues) Swamps native charge of protein Results in average constant charge density AND similar shape for all proteins ...
Protein Purification
... 1. Coomassie Stain: • Denatures protein and binds to hydrophobic core • Excess can be washed away • Detection limit is 0.1 μg ...
... 1. Coomassie Stain: • Denatures protein and binds to hydrophobic core • Excess can be washed away • Detection limit is 0.1 μg ...
A1982NF37500001
... serum albumin had the same emission properties. It turns out that albumin binds nearly everything and is more stable when cornplexed with fatty acids, certain alcohols, or N-acetyltryptophan. These or similar additives are often deliberately incorporated into commercial samples. Impurity removal req ...
... serum albumin had the same emission properties. It turns out that albumin binds nearly everything and is more stable when cornplexed with fatty acids, certain alcohols, or N-acetyltryptophan. These or similar additives are often deliberately incorporated into commercial samples. Impurity removal req ...
Immunohistochemistry for Microsatellite Instability Fact Sheet
... cancer in the future. This information will be helpful in determining the ongoing management of your patient and may also aid in treatment decisions or eligibility for research studies. Patients can benefit from this information by understanding the cause of their cancer, their risk for subsequent c ...
... cancer in the future. This information will be helpful in determining the ongoing management of your patient and may also aid in treatment decisions or eligibility for research studies. Patients can benefit from this information by understanding the cause of their cancer, their risk for subsequent c ...
Protein Sequence Analysis in SeqWEB
... SWISS-PROT excels in annotation, exhibits very little redundancy and is thoroughly integrated with other databases. The extensive annotation and exhaustive to reduce redundancy mean that entries can take time before they are made available, but when they are, they are a complete and thorough resourc ...
... SWISS-PROT excels in annotation, exhibits very little redundancy and is thoroughly integrated with other databases. The extensive annotation and exhaustive to reduce redundancy mean that entries can take time before they are made available, but when they are, they are a complete and thorough resourc ...
Soy protein isolate
... Unlike most other beans, soybeans provide a “complete” protein profile. Soybeans contain all the essential amino acids that we need from our diet, because our bodies are simply not capable of synthesizing them. ...
... Unlike most other beans, soybeans provide a “complete” protein profile. Soybeans contain all the essential amino acids that we need from our diet, because our bodies are simply not capable of synthesizing them. ...
Classification and Regression Tree (CART) Analysis for Deriving
... Many biological processes require change in conformations of proteins, thus, are influenced by the flexibility of the particular protein. This very property of proteins allows a spectrum of interactions between Enzyme-substrate/inhibitor in catalysis and hormone-receptor in biological systems. Thus, ...
... Many biological processes require change in conformations of proteins, thus, are influenced by the flexibility of the particular protein. This very property of proteins allows a spectrum of interactions between Enzyme-substrate/inhibitor in catalysis and hormone-receptor in biological systems. Thus, ...
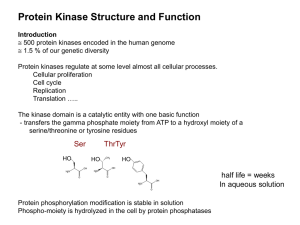
Substrate targeting mechanisms
... Determines Ser/Thr vs Tyrosine specificity and flanking sequences (P+1 loop) ...
... Determines Ser/Thr vs Tyrosine specificity and flanking sequences (P+1 loop) ...
The signal hypothesis matures with age
... Tsirigotaki, and colleagues reveal that bacterial secretory proteins, such as PhoA (pictured), are targeted for secretion by multiple hydrophobic patches (orange) in their mature domains, as well as by their N-terminal signal peptides (green). These mature targeting signals are buried in the fully f ...
... Tsirigotaki, and colleagues reveal that bacterial secretory proteins, such as PhoA (pictured), are targeted for secretion by multiple hydrophobic patches (orange) in their mature domains, as well as by their N-terminal signal peptides (green). These mature targeting signals are buried in the fully f ...
Document
... Sho1p is a protein of 367 amino acids consisting of four predicted transmembrane domains within the Nterminal part, a linker domain, and an SH3 domain for protein-protein interaction. ...
... Sho1p is a protein of 367 amino acids consisting of four predicted transmembrane domains within the Nterminal part, a linker domain, and an SH3 domain for protein-protein interaction. ...
Recombinant polypeptide production inE. coli: towards a rational
... absence of TF the cytoplasmic accumulation of nonproductive protein intermediates would be drastically reduced. Nevertheless, too elevated expression rates would finally result in secretion impairment independently of the deletion strategy and of the chosen export route, since SecB and SRP share the ...
... absence of TF the cytoplasmic accumulation of nonproductive protein intermediates would be drastically reduced. Nevertheless, too elevated expression rates would finally result in secretion impairment independently of the deletion strategy and of the chosen export route, since SecB and SRP share the ...
Computational Molecular Biology 2012
... b) How many of these substitutions are conservative ones according to the default substitution matrix (BLOSUM62) used in BLAST programs for proteins? 7) One of the 8 RNA fragments of the influenza A genome codes for a polymerase called PB1 of about 750 amino acids. It has been recently determined th ...
... b) How many of these substitutions are conservative ones according to the default substitution matrix (BLOSUM62) used in BLAST programs for proteins? 7) One of the 8 RNA fragments of the influenza A genome codes for a polymerase called PB1 of about 750 amino acids. It has been recently determined th ...
Proteome analysis of Arabidopsis thaliana mitochondrial proteins
... One is that fragmentation efficiency of singly charged peptides produced during MALDI post-source decay is enhanced, thereby increasing sensitivity. The other is that only a single fragment ion series (y-ion series) is observed, thereby increasing sensitivity and simplifying the spectrum (applicable ...
... One is that fragmentation efficiency of singly charged peptides produced during MALDI post-source decay is enhanced, thereby increasing sensitivity. The other is that only a single fragment ion series (y-ion series) is observed, thereby increasing sensitivity and simplifying the spectrum (applicable ...
Functional inferences from reconstructed evolutionary biology
... Homologous proteins have analogous folds. Conversely, nonanalogous folds in two protein families indicate that the two families are not homologous. Thus, if two protein families are predicted to have the same fold, they are more likely to share common ancestry. If two protein families are predicted ...
... Homologous proteins have analogous folds. Conversely, nonanalogous folds in two protein families indicate that the two families are not homologous. Thus, if two protein families are predicted to have the same fold, they are more likely to share common ancestry. If two protein families are predicted ...
Peptides
... Polypeptide: a longer peptide with no particular structure Protein: a polypeptide chains with an organized 3D structures The average molecular weight of an amino acid residue is about 110 The molecular weights of most proteins are between 5500 and 220,000 (calculate how many amino acids) We refer to ...
... Polypeptide: a longer peptide with no particular structure Protein: a polypeptide chains with an organized 3D structures The average molecular weight of an amino acid residue is about 110 The molecular weights of most proteins are between 5500 and 220,000 (calculate how many amino acids) We refer to ...
Proteins | Principles of Biology from Nature Education
... © 2011 Nature Education All rights reserved. In Figure 2, notice that the R-groups are categorized according to their ...
... © 2011 Nature Education All rights reserved. In Figure 2, notice that the R-groups are categorized according to their ...
A1989CB63700001
... that time was that average heterozygosity (proportion of heterozygous loci per individual) does not necessarily increase with increasing population size as predicted from the neutral theory and that there is apparently an upper limit of average heterozygosity that is rather low (30 percent). Selecti ...
... that time was that average heterozygosity (proportion of heterozygous loci per individual) does not necessarily increase with increasing population size as predicted from the neutral theory and that there is apparently an upper limit of average heterozygosity that is rather low (30 percent). Selecti ...
amino acid
... of neutral aliphatic amino acids. - Proelastase, the inactive zymogene is cleaved to elastase by trypsin. (4)- Collagenase: it act on proteins present in collagen. (5)- Carboxy peptidase: Two types of carboxy peptidase A and B. The carboxy peptidases are produced as procarboxy peptidases, which are ...
... of neutral aliphatic amino acids. - Proelastase, the inactive zymogene is cleaved to elastase by trypsin. (4)- Collagenase: it act on proteins present in collagen. (5)- Carboxy peptidase: Two types of carboxy peptidase A and B. The carboxy peptidases are produced as procarboxy peptidases, which are ...
Protein folding

Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil.Each protein exists as an unfolded polypeptide or random coil when translated from a sequence of mRNA to a linear chain of amino acids. This polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), known as the native state. The resulting three-dimensional structure is determined by the amino acid sequence (Anfinsen's dogma). Experiments beginning in the 1980s indicate the codon for an amino acid can also influence protein structure.The correct three-dimensional structure is essential to function, although some parts of functional proteins may remain unfolded, so that protein dynamics is important. Failure to fold into native structure generally produces inactive proteins, but in some instances misfolded proteins have modified or toxic functionality. Several neurodegenerative and other diseases are believed to result from the accumulation of amyloid fibrils formed by misfolded proteins. Many allergies are caused by incorrect folding of some proteins, because the immune system does not produce antibodies for certain protein structures.