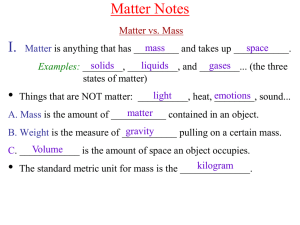
Chem A Week 2 Matter Notes
... of superheated “soup” of bits and pieces of atoms. It only exists ...
... of superheated “soup” of bits and pieces of atoms. It only exists ...
Magnetic Materials Background: 5. Properties
... anisotropy field, Ha (illustrated in figure 4), which is the field required to rotate all the moments by 90° as one unit in a saturated single crystal. The anisotropy is caused by a coupling of the electron orbitals to the lattice, and in the easy direction of magnetisation this coupling is such tha ...
... anisotropy field, Ha (illustrated in figure 4), which is the field required to rotate all the moments by 90° as one unit in a saturated single crystal. The anisotropy is caused by a coupling of the electron orbitals to the lattice, and in the easy direction of magnetisation this coupling is such tha ...
Materials Science in MEMS - Computer Science and Engineering
... heated above a transition temperature (material-dependent) – Ti-Ni most widely used – Can generate very large forces • Good for actuation purposes (unlike piezoelectric and electrostatic actuators, but they can transition much more quickly) ...
... heated above a transition temperature (material-dependent) – Ti-Ni most widely used – Can generate very large forces • Good for actuation purposes (unlike piezoelectric and electrostatic actuators, but they can transition much more quickly) ...
Ch1small - Rutgers University
... • Skim topics in the text before they are covered in lecture. Read the Intro and Summary first. • After lecture, carefully read the topics covered in class. Sample exercises (in the chapter) Practice exercises (at end of chapter) ...
... • Skim topics in the text before they are covered in lecture. Read the Intro and Summary first. • After lecture, carefully read the topics covered in class. Sample exercises (in the chapter) Practice exercises (at end of chapter) ...
Chapter 3
... electron can have and how likely it is to find the electron in various locations around the nucleus of an atom. ...
... electron can have and how likely it is to find the electron in various locations around the nucleus of an atom. ...
Statistical Mechanics of Phase Transition
... Under this scheme phase transitions are labeled by the lowest derivative of the free energy that is discontinuous at the transition. First order phase transition exhibits discontinuity in the 1st derivative of the free energy with a thermodynamic variable. Ex.- solid/liquid/gas transitions. Second o ...
... Under this scheme phase transitions are labeled by the lowest derivative of the free energy that is discontinuous at the transition. First order phase transition exhibits discontinuity in the 1st derivative of the free energy with a thermodynamic variable. Ex.- solid/liquid/gas transitions. Second o ...
I2(10 o) - Rutgers Physics
... (c) (4 points) There are two long wires, separated by 1 m. One carries 2 A of current and the other caries 3 A of current in the same direction as the first wire. Find the magnitude of the net magnetic field half way between the two wires. Solution: A point that is half way between the wires is 0.5 ...
... (c) (4 points) There are two long wires, separated by 1 m. One carries 2 A of current and the other caries 3 A of current in the same direction as the first wire. Find the magnitude of the net magnetic field half way between the two wires. Solution: A point that is half way between the wires is 0.5 ...
Ask a scientist answers
... Q11: “Since beta decay is when …” A11: Good question. The weak force is responsible for changing one quark into another, in this case a d-quark into a u-quark. Because the electric charge must conserved one needs to emit a negative charge (the neutron is neutral, the proton is +1, so you need a -1ty ...
... Q11: “Since beta decay is when …” A11: Good question. The weak force is responsible for changing one quark into another, in this case a d-quark into a u-quark. Because the electric charge must conserved one needs to emit a negative charge (the neutron is neutral, the proton is +1, so you need a -1ty ...
Matter and Atoms
... How atoms combine – 3.2 Objectives • Describe the chemical bonds that unit atoms to ...
... How atoms combine – 3.2 Objectives • Describe the chemical bonds that unit atoms to ...
Powerpoint
... I personally find the three-fingered axis system to often (but not always) be the most useful way to apply the right-hand rule. ...
... I personally find the three-fingered axis system to often (but not always) be the most useful way to apply the right-hand rule. ...
PowerPoint
... I personally find the three-fingered axis system to often (but not always) be the most useful way to apply the right-hand rule. ...
... I personally find the three-fingered axis system to often (but not always) be the most useful way to apply the right-hand rule. ...
Intensive properties
... • Other properties that are extensive would include size, shape, etc. • Intensive properties are ones that only depend on what kind of stuff you have, like color, etc. ...
... • Other properties that are extensive would include size, shape, etc. • Intensive properties are ones that only depend on what kind of stuff you have, like color, etc. ...
Motion of Charged Particles in Magnetic Fields File
... Perpendicular to E and directed into the page is a magnetic field B = 0.4 T. (b) Write down an expression for the magnetic force acting on the charge in terms of ‘q’, the speed ‘v’ and the magnetic field strength given. If the speed of the particles is properly chosen, the force due to the electric ...
... Perpendicular to E and directed into the page is a magnetic field B = 0.4 T. (b) Write down an expression for the magnetic force acting on the charge in terms of ‘q’, the speed ‘v’ and the magnetic field strength given. If the speed of the particles is properly chosen, the force due to the electric ...
Unit 1 – Matter and Change
... • Compounds – Made up of two or more types of atoms – Chemically bonded – Can be broken down into simple, stable substances • Must be chemical separation, not physical separation – Ex: water (H2O), sugar (C12H22O11), salt (NaCl), etc. ...
... • Compounds – Made up of two or more types of atoms – Chemically bonded – Can be broken down into simple, stable substances • Must be chemical separation, not physical separation – Ex: water (H2O), sugar (C12H22O11), salt (NaCl), etc. ...
File
... 20. The rows in the periodic table are called ____________________. They are organized by ______________________________________. 21. The columns in the periodic table are called ______________________. They are grouped by ____________________________________________________________. 22. Most of the ...
... 20. The rows in the periodic table are called ____________________. They are organized by ______________________________________. 21. The columns in the periodic table are called ______________________. They are grouped by ____________________________________________________________. 22. Most of the ...
Nuclear and Radiation Section - University of Toronto Physics
... The value of Z determines the chemical behaviour of the element. However different nuclei can have the same value of Z, yet different values of N (and thus of A). Nuclei with the same Z but different A values are called isotopes. Thus 12C, 11C, and 14C are isotopes of Carbon. Isotopes are either sta ...
... The value of Z determines the chemical behaviour of the element. However different nuclei can have the same value of Z, yet different values of N (and thus of A). Nuclei with the same Z but different A values are called isotopes. Thus 12C, 11C, and 14C are isotopes of Carbon. Isotopes are either sta ...
MeasurementReview
... It is suggested that the relationship between v and λ is of the form v= a where a is a constant. To test the validity of this hypothesis, values of v2 against λ are plotted below. ...
... It is suggested that the relationship between v and λ is of the form v= a where a is a constant. To test the validity of this hypothesis, values of v2 against λ are plotted below. ...
Condensed matter physics

Condensed matter physics is a branch of physics that deals with the physical properties of condensed phases of matter. Condensed matter physicists seek to understand the behavior of these phases by using physical laws. In particular, these include the laws of quantum mechanics, electromagnetism and statistical mechanics.The most familiar condensed phases are solids and liquids, while more exotic condensed phases include the superconducting phase exhibited by certain materials at low temperature, the ferromagnetic and antiferromagnetic phases of spins on atomic lattices, and the Bose–Einstein condensate found in cold atomic systems. The study of condensed matter physics involves measuring various material properties via experimental probes along with using techniques of theoretical physics to develop mathematical models that help in understanding physical behavior.The diversity of systems and phenomena available for study makes condensed matter physics the most active field of contemporary physics: one third of all American physicists identify themselves as condensed matter physicists, and the Division of Condensed Matter Physics is the largest division at the American Physical Society. The field overlaps with chemistry, materials science, and nanotechnology, and relates closely to atomic physics and biophysics. Theoretical condensed matter physics shares important concepts and techniques with theoretical particle and nuclear physics.A variety of topics in physics such as crystallography, metallurgy, elasticity, magnetism, etc., were treated as distinct areas, until the 1940s when they were grouped together as solid state physics. Around the 1960s, the study of physical properties of liquids was added to this list, forming the basis for the new, related specialty of condensed matter physics. According to physicist Phil Anderson, the term was coined by him and Volker Heine when they changed the name of their group at the Cavendish Laboratories, Cambridge from ""Solid state theory"" to ""Theory of Condensed Matter"" in 1967, as they felt it did not exclude their interests in the study of liquids, nuclear matter and so on. Although Anderson and Heine helped popularize the name ""condensed matter"", it had been present in Europe for some years, most prominently in the form of a journal published in English, French, and German by Springer-Verlag titled Physics of Condensed Matter, which was launched in 1963. The funding environment and Cold War politics of the 1960s and 1970s were also factors that lead some physicists to prefer the name ""condensed matter physics"", which emphasized the commonality of scientific problems encountered by physicists working on solids, liquids, plasmas, and other complex matter, over ""solid state physics"", which was often associated with the industrial applications of metals and semiconductors. The Bell Telephone Laboratories was one of the first institutes to conduct a research program in condensed matter physics.References to ""condensed"" state can be traced to earlier sources. For example, in the introduction to his 1947 ""Kinetic theory of liquids"" book, Yakov Frenkel proposed that ""The kinetic theory of liquids must accordingly be developed as a generalization and extension of the kinetic theory of solid bodies"". As a matter of fact, it would be more correct to unify them under the title of ""condensed bodies"".























