Guidelines for Cosmetic Advertising and Labelling Claims
... The Guidelines are designed to help creators of advertising develop advertising messages, including those made on or inside the package, that comply with Canadian cosmetic regulatory requirements. The publication of the Guidelines represents the culmination of a collaborative effort between Advertis ...
... The Guidelines are designed to help creators of advertising develop advertising messages, including those made on or inside the package, that comply with Canadian cosmetic regulatory requirements. The publication of the Guidelines represents the culmination of a collaborative effort between Advertis ...
Interactions between the cytochrome P450 system and
... 48.5% inhibition of clozapine metabolism; 42.0% inhibition was observed with the chemical CYP1A2 furafyline. Similarly, Olesen and Linnet42 noted significant inhibition of clozapine demethylation in vitro after incubation with fluvoxamine, a reaction catalyzed in vitro by CYP1A2, CYP2C9, CYP2C19, CY ...
... 48.5% inhibition of clozapine metabolism; 42.0% inhibition was observed with the chemical CYP1A2 furafyline. Similarly, Olesen and Linnet42 noted significant inhibition of clozapine demethylation in vitro after incubation with fluvoxamine, a reaction catalyzed in vitro by CYP1A2, CYP2C9, CYP2C19, CY ...
Master-Thesis
... Counterfeit medicines are present in industrialized and developing countries. The extent of the problem and several other factors concerning the counterfeited drugs however differ significantly between industrialized and developing countries. E.g., the market share of counterfeit drugs is below 1% o ...
... Counterfeit medicines are present in industrialized and developing countries. The extent of the problem and several other factors concerning the counterfeited drugs however differ significantly between industrialized and developing countries. E.g., the market share of counterfeit drugs is below 1% o ...
Synagis (palivizumab) SAMPLE CMS 1500 CLAIM FORM Box 19
... Animal Data Animal reproduction studies have not been conducted. ...
... Animal Data Animal reproduction studies have not been conducted. ...
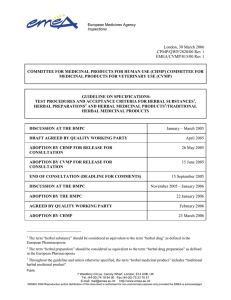
guideline on specificatons: test procedures and acceptance
... reflects the current state of the art at the time it has been written, and should not be considered allencompassing. New analytical technologies, and modifications to existing technologies, are continuously being developed. Such technologies should be used when appropriate. ...
... reflects the current state of the art at the time it has been written, and should not be considered allencompassing. New analytical technologies, and modifications to existing technologies, are continuously being developed. Such technologies should be used when appropriate. ...
Telfast 180mg
... been administered to healthy subjects without the development of clinically significant adverse events as compared with placebo. The maximum tolerated dose of fexofenadine hydrochloride has not been established. Standard measures should be considered to remove any unabsorbed drug. Symptomatic and su ...
... been administered to healthy subjects without the development of clinically significant adverse events as compared with placebo. The maximum tolerated dose of fexofenadine hydrochloride has not been established. Standard measures should be considered to remove any unabsorbed drug. Symptomatic and su ...
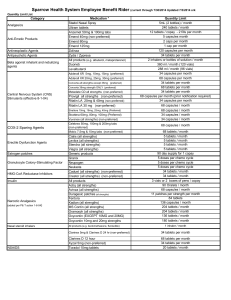
Benefit Rider - Sparrow Health System
... Xolegel DUO and Corepak Xyralid products Zamicet Zenieva Z-Care Zinotic Zinx Zotex-D Z Tuss 2 Zypram Zytaze Zytopic ...
... Xolegel DUO and Corepak Xyralid products Zamicet Zenieva Z-Care Zinotic Zinx Zotex-D Z Tuss 2 Zypram Zytaze Zytopic ...
Advances in phage display technology for drug discovery
... replication, the replicative form produces ss-DNA and also serves as template for phage protein expression. The C-terminal domain anchors the pIII in phage coat by interacting with other phage coat proteins. Therefore, it is responsible for the integration of pIII into the phage coat. The C-terminal ...
... replication, the replicative form produces ss-DNA and also serves as template for phage protein expression. The C-terminal domain anchors the pIII in phage coat by interacting with other phage coat proteins. Therefore, it is responsible for the integration of pIII into the phage coat. The C-terminal ...
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS)
... formulations are dumped in Indian market. If it continues so on ,pubic will suffer with drug induced health hazards in future. Accepted formulations are only 19.6%.we have to encourage these drugs for safety of patients. As these are cheaper than that of irrational and miscellaneous formulations ,th ...
... formulations are dumped in Indian market. If it continues so on ,pubic will suffer with drug induced health hazards in future. Accepted formulations are only 19.6%.we have to encourage these drugs for safety of patients. As these are cheaper than that of irrational and miscellaneous formulations ,th ...
user guide - Micromedex
... This manual, as well as the data and software implementation described in it, is furnished under license and may be used or copied only in accordance with the terms of such license. The content of this manual is furnished for informational use only, is subject to change without notice, and should no ...
... This manual, as well as the data and software implementation described in it, is furnished under license and may be used or copied only in accordance with the terms of such license. The content of this manual is furnished for informational use only, is subject to change without notice, and should no ...
Micromedex User guide
... This manual, as well as the data and software implementation described in it, is furnished under license and may be used or copied only in accordance with the terms of such license. The content of this manual is furnished for informational use only, is subject to change without notice, and should no ...
... This manual, as well as the data and software implementation described in it, is furnished under license and may be used or copied only in accordance with the terms of such license. The content of this manual is furnished for informational use only, is subject to change without notice, and should no ...
Data Analytics with SNOMED CT â Case Studies
... In this collaborative project, clinical data is being represented in OWL-RL as ‘entity-role-act’ triples. This uses a logical model (with Entities in Roles participating in Acts) that is similar to HL7 V3’s Reference Information Model. OWL-RL and Datalog rule language is being used to reason over hu ...
... In this collaborative project, clinical data is being represented in OWL-RL as ‘entity-role-act’ triples. This uses a logical model (with Entities in Roles participating in Acts) that is similar to HL7 V3’s Reference Information Model. OWL-RL and Datalog rule language is being used to reason over hu ...
Tybost - Gilead Sciences, Inc.
... interactions. TYBOST and ritonavir when administered with either atazanavir or darunavir may result in different drug interactions when used with concomitant medications. (5.3, 7, 12.3). ------------------------DOSAGE AND ADMINISTRATION --------------------- TYBOST must be coadministered with ataza ...
... interactions. TYBOST and ritonavir when administered with either atazanavir or darunavir may result in different drug interactions when used with concomitant medications. (5.3, 7, 12.3). ------------------------DOSAGE AND ADMINISTRATION --------------------- TYBOST must be coadministered with ataza ...
Analysis Reveals that CPX-351 Shifts the Exposure of Cytarabine
... preclinically. Clinically, CPX-351 has provided evidence of promising improvements in patient outcomes, most notably in elderly newly diagnosed high risk (secondary) AML and in unfavorable risk first relapse adult AML where statistically significant increases in overall survival where observed in tw ...
... preclinically. Clinically, CPX-351 has provided evidence of promising improvements in patient outcomes, most notably in elderly newly diagnosed high risk (secondary) AML and in unfavorable risk first relapse adult AML where statistically significant increases in overall survival where observed in tw ...
Inhibition and induction of human cytochrome P450 (CYP) enzymes
... their quantitation in in vitro incubations, it is possible to employ ` diagnostic ’ inhibitors (table 2) and to look which of them, and at which concentrations, inhibit metabolic routes. It is also possible to use enzyme-speci® c antibodies and to test which metabolic routes are inhibited and to wha ...
... their quantitation in in vitro incubations, it is possible to employ ` diagnostic ’ inhibitors (table 2) and to look which of them, and at which concentrations, inhibit metabolic routes. It is also possible to use enzyme-speci® c antibodies and to test which metabolic routes are inhibited and to wha ...
If your drug is not “on the list” just give us a call for a price. Ask us for
... We CANNOT substitute any prescription (or refill) written for Viagra® without a phone call to the prescriber to get authorization for the following New Rx: Rx ...
... We CANNOT substitute any prescription (or refill) written for Viagra® without a phone call to the prescriber to get authorization for the following New Rx: Rx ...