TEST on Atomic Structure
... ____ 41) Which of the following is true about the composition of ionic compounds? a. They are composed of anions and cations. c. They are composed of cations only. b. They are composed of anions only. d. They are formed from two or more nonmetallic elements. ____ 42) Which element, when combined wit ...
... ____ 41) Which of the following is true about the composition of ionic compounds? a. They are composed of anions and cations. c. They are composed of cations only. b. They are composed of anions only. d. They are formed from two or more nonmetallic elements. ____ 42) Which element, when combined wit ...
UNIT GUIDES 2014-2015 FUNDAMENTALS OF PHYSICS IN ENGINEERING I
... primarily between ionic, covalent and metallic solids, and introducing the free- electron model of metals, the concept of density of states and Fermi-Dirac’s distribution. We analyse the difference between conductors, insulators and semiconductors based on their energy band structure and the separat ...
... primarily between ionic, covalent and metallic solids, and introducing the free- electron model of metals, the concept of density of states and Fermi-Dirac’s distribution. We analyse the difference between conductors, insulators and semiconductors based on their energy band structure and the separat ...
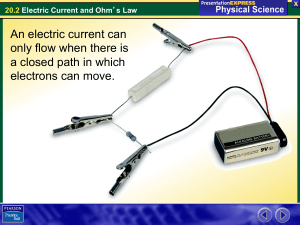
20.2 Electric Current and Ohm
... • Each ion has one or more electrons that are not tightly bound to it. • These free electrons can conduct charge. • Most materials do not easily conduct charge because they don’t have free electrons. ...
... • Each ion has one or more electrons that are not tightly bound to it. • These free electrons can conduct charge. • Most materials do not easily conduct charge because they don’t have free electrons. ...
Chapter 3: semiconductor science and light emitting diodes
... ¾ Broadening of allowed energy levels into allowed energy bands separated by forbidden-energy gaps as more atoms influence each electron in a solid. ...
... ¾ Broadening of allowed energy levels into allowed energy bands separated by forbidden-energy gaps as more atoms influence each electron in a solid. ...
Sci-Fi Helper - Magnetic Fields
... Section: Properties of Electric Charges www.encyclopedia.com/html/section/electity_propertiesofelectriccharges.asp According to modern theory, most elementary particles of matter possess charge, either positive or negative. Two particles with like charges, both positive or both negative, repel each ...
... Section: Properties of Electric Charges www.encyclopedia.com/html/section/electity_propertiesofelectriccharges.asp According to modern theory, most elementary particles of matter possess charge, either positive or negative. Two particles with like charges, both positive or both negative, repel each ...
Magnetic Fields
... Section: Properties of Electric Charges www.encyclopedia.com/html/section/electity_propertiesofelectriccharges.asp According to modern theory, most elementary particles of matter possess charge, either positive or negative. Two particles with like charges, both positive or both negative, repel each ...
... Section: Properties of Electric Charges www.encyclopedia.com/html/section/electity_propertiesofelectriccharges.asp According to modern theory, most elementary particles of matter possess charge, either positive or negative. Two particles with like charges, both positive or both negative, repel each ...
Unit 14.1 REDOX Reactions Objectives REDOX Reactions
... • REDOX reactions can be used to convert chemical potential energy into electrical energy. ...
... • REDOX reactions can be used to convert chemical potential energy into electrical energy. ...
Lecture 5
... The conductor has an excess of positive charge All of the charge resides at the surface E = 0 inside the conductor The electric field just outside the conductor is perpendicular to the surface The potential is a constant everywhere on the surface of the conductor The potential everywhere inside the ...
... The conductor has an excess of positive charge All of the charge resides at the surface E = 0 inside the conductor The electric field just outside the conductor is perpendicular to the surface The potential is a constant everywhere on the surface of the conductor The potential everywhere inside the ...
2010
... (i) What is meant by refraction of light? (ii) What is the cause of refraction of light? 'The refractive index of diamond is 2.42'. What is meant by this statement? We can burn a piece of paper by focussing the sun rays by using a particular type of lens. (i) Name the type of lens used for the above ...
... (i) What is meant by refraction of light? (ii) What is the cause of refraction of light? 'The refractive index of diamond is 2.42'. What is meant by this statement? We can burn a piece of paper by focussing the sun rays by using a particular type of lens. (i) Name the type of lens used for the above ...
Ch16_2008
... •Field thus points toward a negative charge and away from a positive charge •Since test charge is positive, the direction of the electric field is the direction of the force felt by a positive charge •If there are two or more charges creating the field then the field at any point is the vector sum o ...
... •Field thus points toward a negative charge and away from a positive charge •Since test charge is positive, the direction of the electric field is the direction of the force felt by a positive charge •If there are two or more charges creating the field then the field at any point is the vector sum o ...
First Semester complete review with answers
... Elements in each row have the same number of electron shells 32. What do elements in the same column/group/family have in common? Elements in each group have the same number of valence electrons and thus have similar reactivity. ...
... Elements in each row have the same number of electron shells 32. What do elements in the same column/group/family have in common? Elements in each group have the same number of valence electrons and thus have similar reactivity. ...