PHYS 110A - HW #8
... reaches the speed of light. This serves as another example that magnetic effects are truly a relativistic phenomena and are generally insignificant compared to electric effects. [7.] Problem 5.19 from Griffiths (a) Find the density of free charges in copper. Assume that each copper atom contributes ...
... reaches the speed of light. This serves as another example that magnetic effects are truly a relativistic phenomena and are generally insignificant compared to electric effects. [7.] Problem 5.19 from Griffiths (a) Find the density of free charges in copper. Assume that each copper atom contributes ...
Spring Semester Jeopardy
... If an object A is at rest and is struck by object B and a perfectly elastic collision occurs, what is the speed of Object B after the collision Answer ...
... If an object A is at rest and is struck by object B and a perfectly elastic collision occurs, what is the speed of Object B after the collision Answer ...
Ohm`s Law - DigitalCommons@University of Nebraska
... Read Chapter 27, Sections 27-1 through 27-6. The definition of current is qiven by Eq. (27-1), and a very thorough discussion follows in Section 27-1. A relation for current density is given in Eq. (27-5), and a more general inteqral form is given in Eq. (27-7). Read General Comments 1 and 2. The co ...
... Read Chapter 27, Sections 27-1 through 27-6. The definition of current is qiven by Eq. (27-1), and a very thorough discussion follows in Section 27-1. A relation for current density is given in Eq. (27-5), and a more general inteqral form is given in Eq. (27-7). Read General Comments 1 and 2. The co ...
Simulation of discharging dust grains by laser excitation of neutral...
... weakly bound electron by electric fields that depend on the size of the binding energy. For atoms in a state with principle quantum number n, the field to strip the electron is approximately Fstrip ⬃ 3.2⫻ 108 V / cm/ n4. The field rapidly decreases with increasing n; this field is ⬃2 kV/ cm for n = ...
... weakly bound electron by electric fields that depend on the size of the binding energy. For atoms in a state with principle quantum number n, the field to strip the electron is approximately Fstrip ⬃ 3.2⫻ 108 V / cm/ n4. The field rapidly decreases with increasing n; this field is ⬃2 kV/ cm for n = ...
Capacitors
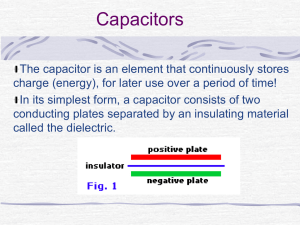
... The capacitor is an element that continuously stores charge (energy), for later use over a period of time! In its simplest form, a capacitor consists of two conducting plates separated by an insulating material called the dielectric. ...
... The capacitor is an element that continuously stores charge (energy), for later use over a period of time! In its simplest form, a capacitor consists of two conducting plates separated by an insulating material called the dielectric. ...
once upon a time, there was electricity
... In 1831, the ubiquitous Michael Faraday showed that it is possible to create electric current in a closed coil when a magnetic bar is introduced and then withdrawn. This marked the discovery of the principle of induction, the basis of the functioning of electricity generators, the first models of wh ...
... In 1831, the ubiquitous Michael Faraday showed that it is possible to create electric current in a closed coil when a magnetic bar is introduced and then withdrawn. This marked the discovery of the principle of induction, the basis of the functioning of electricity generators, the first models of wh ...
SM_chapter19
... two particles, and inversely proportional to the square of the separation distance. An electrical force exhibits the same proportionalities, with charge as the intrinsic property. Differences: The electrical force can either attract or repel, while the gravitational force as described by Newton’s la ...
... two particles, and inversely proportional to the square of the separation distance. An electrical force exhibits the same proportionalities, with charge as the intrinsic property. Differences: The electrical force can either attract or repel, while the gravitational force as described by Newton’s la ...
Battery Charging Terminology
... The Ah or Ampere/hour capacity is the current a battery can provide over a specified period of time, e.g. 100Ah @ C10 rate to EOD of 1.75V/cell. This means the battery can provide 10 Amps for 10 hours to an end of discharge voltage of 1.75V per cell. Different battery manufacturers will use differen ...
... The Ah or Ampere/hour capacity is the current a battery can provide over a specified period of time, e.g. 100Ah @ C10 rate to EOD of 1.75V/cell. This means the battery can provide 10 Amps for 10 hours to an end of discharge voltage of 1.75V per cell. Different battery manufacturers will use differen ...
The Scattering of α and β Particles by Matter and the
... probable angle of deflexions for a pencil of α particles traversing a gold–foil of this thickness was about 0.87◦ . A simple calculation based on the theory of probability shows that the chance of an α particle being deflected through 90 degrees is vanishingly small. In addition, it will be seen la ...
... probable angle of deflexions for a pencil of α particles traversing a gold–foil of this thickness was about 0.87◦ . A simple calculation based on the theory of probability shows that the chance of an α particle being deflected through 90 degrees is vanishingly small. In addition, it will be seen la ...
Electric charge
Electric charge is the physical property of matter that causes it to experience a force when placed in an electromagnetic field. There are two types of electric charges: positive and negative. Positively charged substances are repelled from other positively charged substances, but attracted to negatively charged substances; negatively charged substances are repelled from negative and attracted to positive. An object is negatively charged if it has an excess of electrons, and is otherwise positively charged or uncharged. The SI derived unit of electric charge is the coulomb (C), although in electrical engineering it is also common to use the ampere-hour (Ah), and in chemistry it is common to use the elementary charge (e) as a unit. The symbol Q is often used to denote charge. The early knowledge of how charged substances interact is now called classical electrodynamics, and is still very accurate if quantum effects do not need to be considered.The electric charge is a fundamental conserved property of some subatomic particles, which determines their electromagnetic interaction. Electrically charged matter is influenced by, and produces, electromagnetic fields. The interaction between a moving charge and an electromagnetic field is the source of the electromagnetic force, which is one of the four fundamental forces (See also: magnetic field).Twentieth-century experiments demonstrated that electric charge is quantized; that is, it comes in integer multiples of individual small units called the elementary charge, e, approximately equal to 6981160200000000000♠1.602×10−19 coulombs (except for particles called quarks, which have charges that are integer multiples of e/3). The proton has a charge of +e, and the electron has a charge of −e. The study of charged particles, and how their interactions are mediated by photons, is called quantum electrodynamics.