The Sanity Project A Survival Guide and Celebration of Homeless
... here, but the feds say we need to hire someone to go look for them.” Homeless liaisons are the only one doing what we do in the district. Many of us do not have other staff. ...
... here, but the feds say we need to hire someone to go look for them.” Homeless liaisons are the only one doing what we do in the district. Many of us do not have other staff. ...
Name: Magnetic Field and Lorentz Force
... 3. An electron accelerated from rest through potential difference V1 = 1.00 kV enters the gap between two parallel plates having separation d = 20.0 mm and potential difference V2 = 100 V. The lower plate is at the lower potential. Neglect fringing and assume that the electron's velocity vector is p ...
... 3. An electron accelerated from rest through potential difference V1 = 1.00 kV enters the gap between two parallel plates having separation d = 20.0 mm and potential difference V2 = 100 V. The lower plate is at the lower potential. Neglect fringing and assume that the electron's velocity vector is p ...
Chemistry 102 Summary June 25th - Bohr model only works for one
... Orbitals define the allowed energy states where electrons can reside. There are four basic shapes: s, p, d and f Shapes represent where an electron will reside 90 % of the time in that allowed energy state. From Heisenberg – the exact location cannot be determined but instead the probability (Ψ2). E ...
... Orbitals define the allowed energy states where electrons can reside. There are four basic shapes: s, p, d and f Shapes represent where an electron will reside 90 % of the time in that allowed energy state. From Heisenberg – the exact location cannot be determined but instead the probability (Ψ2). E ...
Quantum Theory Historical Reference
... Ultimately explains the quantized energy of electrons. de Broglie = h/(mv) h = Plank’s constant: 6.63 x 10-34 J.s In order to observe the wave nature of matter, the de Broglie must be large such that it is measurable. Only fundamental particles (extremely small masses) have such ’s and obey ...
... Ultimately explains the quantized energy of electrons. de Broglie = h/(mv) h = Plank’s constant: 6.63 x 10-34 J.s In order to observe the wave nature of matter, the de Broglie must be large such that it is measurable. Only fundamental particles (extremely small masses) have such ’s and obey ...
Presentation
... 379000 years after the big bang when the universe became transparent to electromagnetic radiation, what was the peak wavelength of the curve? 976 nm ...
... 379000 years after the big bang when the universe became transparent to electromagnetic radiation, what was the peak wavelength of the curve? 976 nm ...
Document
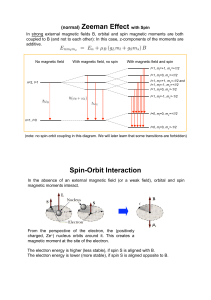
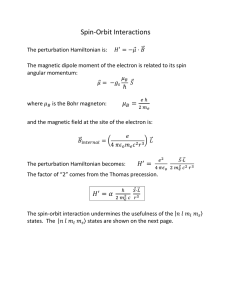
... Energy depends on L and S, not on ML or MS. • (L, S, J, MJ) are good quantum numbers for heavy many-electron atoms with significant spin-orbit coupling (relativistic effect). Energy also depends on J. • For very heavy atoms, a j-j coupling is needed, where j = l + s for each electron. ...
... Energy depends on L and S, not on ML or MS. • (L, S, J, MJ) are good quantum numbers for heavy many-electron atoms with significant spin-orbit coupling (relativistic effect). Energy also depends on J. • For very heavy atoms, a j-j coupling is needed, where j = l + s for each electron. ...
Atomic Emission Spectra – Copy
... • We can turn a continuous emission spectra into a discontinuous one by refracting the light. • An Atomic Emission Spectrum is a set of frequencies of electromagnetic waves emitted by atoms of the element. They are usually distinct color lines. Each element’s atomic emission spectrum is unique, can ...
... • We can turn a continuous emission spectra into a discontinuous one by refracting the light. • An Atomic Emission Spectrum is a set of frequencies of electromagnetic waves emitted by atoms of the element. They are usually distinct color lines. Each element’s atomic emission spectrum is unique, can ...
Phys202_Final_Exam_Spr2006.doc
... The +z direction is out of paper toward your face and +x is to your right, +y up the page. IGNORE the sign of your answer and select the correct magnitude from the list. You may not leave prior the then end of the class after all papers are collected. You may only have pencils and a one memory non-p ...
... The +z direction is out of paper toward your face and +x is to your right, +y up the page. IGNORE the sign of your answer and select the correct magnitude from the list. You may not leave prior the then end of the class after all papers are collected. You may only have pencils and a one memory non-p ...
Exam #: _____________________ Printed Name: ________________ Signature:___________________ PHYSICS DEPARTMENT
... sign your name in the spaces provided on this page. For identification purposes, be sure to submit this page together with your answers when the exam is finished. Be sure to place both the exam number and the question number on any additional pages you wish to have graded. There are six equally weig ...
... sign your name in the spaces provided on this page. For identification purposes, be sure to submit this page together with your answers when the exam is finished. Be sure to place both the exam number and the question number on any additional pages you wish to have graded. There are six equally weig ...
11/14 Lecture outline • Binomial distribution: recall p(N1) = ( N N1
... expand it in x, around x = 1/2, and keep just the lowest order term involving x: ...
... expand it in x, around x = 1/2, and keep just the lowest order term involving x: ...
Klicker-questions, chapter 1 1. The figure shows the probability
... 3. Assume the wave function of a particle is given by Ψ ( x, t ) = ei (kx −ωt ) If you measure the position of the particle where is the largest probability to find it? a) Around x=0. b) Depends of the time t. c) The probability to find the particle is the same everywhere. 4. The probability distrib ...
... 3. Assume the wave function of a particle is given by Ψ ( x, t ) = ei (kx −ωt ) If you measure the position of the particle where is the largest probability to find it? a) Around x=0. b) Depends of the time t. c) The probability to find the particle is the same everywhere. 4. The probability distrib ...
Electrons in Atoms
... A. Based on mathematical equation (previous models were basically physical) B. Concerned with predicting the probable location of electrons 1. when all the possible mathematical solutions are graphed, a 3-D shape results (a "cloud" of probability) (orbital) 2. although drawn spherical, atom is not n ...
... A. Based on mathematical equation (previous models were basically physical) B. Concerned with predicting the probable location of electrons 1. when all the possible mathematical solutions are graphed, a 3-D shape results (a "cloud" of probability) (orbital) 2. although drawn spherical, atom is not n ...
Electrons in Atoms
... A. Based on mathematical equation (previous models were basically physical) B. Concerned with predicting the probable location of electrons 1. when all the possible mathematical solutions are graphed, a 3-D shape results (a "cloud" of probability) (orbital) 2. although drawn spherical, atom is not n ...
... A. Based on mathematical equation (previous models were basically physical) B. Concerned with predicting the probable location of electrons 1. when all the possible mathematical solutions are graphed, a 3-D shape results (a "cloud" of probability) (orbital) 2. although drawn spherical, atom is not n ...
Chapter 7 Many-Electron Atoms
... also important in magnetism. Spectral lines are due to photons emitted when electrons change their energy state. Changes in the principal quantum number n cause the most noticeable changes. However, changes in other quantum numbers also give rise to changes in electron energies. Such changes typical ...
... also important in magnetism. Spectral lines are due to photons emitted when electrons change their energy state. Changes in the principal quantum number n cause the most noticeable changes. However, changes in other quantum numbers also give rise to changes in electron energies. Such changes typical ...
Quantum electrodynamics

In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it ""the jewel of physics"" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen.