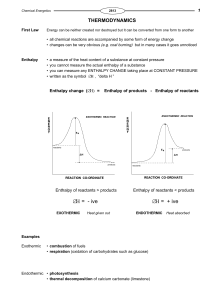
Basics of Chemistry
... Some elements exist in more than one form. Familiar examples include (1) oxygen, found as O2 molecules, and ozone, found as O3 molecules (fig. 8) and (2) two different crystalline forms of carbon—diamond and graphite. Different forms of the same element in the same physical state are called allotrop ...
... Some elements exist in more than one form. Familiar examples include (1) oxygen, found as O2 molecules, and ozone, found as O3 molecules (fig. 8) and (2) two different crystalline forms of carbon—diamond and graphite. Different forms of the same element in the same physical state are called allotrop ...
Fundamentals
... known that atoms are composed of yet smaller particles, an atom remains the smallest entity that an element can be broken down into and retain the properties of that element. Atoms can be imaged with two types of instruments: a scanning tunneling microscope (STM) and an atomic force microscope (AFM) ...
... known that atoms are composed of yet smaller particles, an atom remains the smallest entity that an element can be broken down into and retain the properties of that element. Atoms can be imaged with two types of instruments: a scanning tunneling microscope (STM) and an atomic force microscope (AFM) ...
Examples
... mass of one mole of any substance (expressed in grams/mol) The same as: 1) Gram Molecular Mass (for molecules) 2) Gram Formula Mass (ionic compounds) 3) Gram Atomic Mass (for elements) – molar mass is just a much broader term than these other specific masses ...
... mass of one mole of any substance (expressed in grams/mol) The same as: 1) Gram Molecular Mass (for molecules) 2) Gram Formula Mass (ionic compounds) 3) Gram Atomic Mass (for elements) – molar mass is just a much broader term than these other specific masses ...
Metalloid Al- and Ga-clusters: a novel dimension in organometallic
... this process of the formation and breaking of metal–metal bonds (MM) are mostly unknown. This fundamental process and molecular intermediates exhibiting MM bonding are therefore central to this contribution. These molecular intermediates are mostly addressed as metal atom clusters.1 However, since t ...
... this process of the formation and breaking of metal–metal bonds (MM) are mostly unknown. This fundamental process and molecular intermediates exhibiting MM bonding are therefore central to this contribution. These molecular intermediates are mostly addressed as metal atom clusters.1 However, since t ...
Slide 1
... I. Atomic Mass A. Relative Mass is when you measure the mass of all atoms based on the measurement of one particular atom. ...
... I. Atomic Mass A. Relative Mass is when you measure the mass of all atoms based on the measurement of one particular atom. ...
Chapter 2
... • Because in the real world we use large amounts of atoms and molecules, we use average masses in calculations. • Average mass is calculated from the isotopes of an element weighted by their relative abundances. Atoms, Molecules, and Ions ...
... • Because in the real world we use large amounts of atoms and molecules, we use average masses in calculations. • Average mass is calculated from the isotopes of an element weighted by their relative abundances. Atoms, Molecules, and Ions ...
Chapter 2 ppt - Renton School District
... • Because in the real world we use large amounts of atoms and molecules, we use average masses in calculations. • Average mass is calculated from the isotopes of an element weighted by their relative abundances. Atoms, Molecules, and Ions ...
... • Because in the real world we use large amounts of atoms and molecules, we use average masses in calculations. • Average mass is calculated from the isotopes of an element weighted by their relative abundances. Atoms, Molecules, and Ions ...
Chapter 7
... • Thomson determined the mass-to-charge ratio; Millikan found the charge; we can now find the mass of an electron: ...
... • Thomson determined the mass-to-charge ratio; Millikan found the charge; we can now find the mass of an electron: ...
chapter_4_notes
... Was a young Danish physicist and a student of Rutherford’s when he proposed that the electron is only found in specific circular paths, or orbits, around the nucleus. This model of the atom is similar to the solar system because the electrons orbit the nucleus like the planets orbit the sun. Each el ...
... Was a young Danish physicist and a student of Rutherford’s when he proposed that the electron is only found in specific circular paths, or orbits, around the nucleus. This model of the atom is similar to the solar system because the electrons orbit the nucleus like the planets orbit the sun. Each el ...
Practice Exam I FR Answers and Explanations
... Cd changes oxidation states from 0 to +2—thus, it is oxidized. Whatever species is oxidized is known as the reducing agent. (c) At a higher temperature, how would the cell potential change? Explain questions such as this with mathematical formulas if at all possible. There are two equations that all ...
... Cd changes oxidation states from 0 to +2—thus, it is oxidized. Whatever species is oxidized is known as the reducing agent. (c) At a higher temperature, how would the cell potential change? Explain questions such as this with mathematical formulas if at all possible. There are two equations that all ...
SCHLOSS RINGBERG
... the nitric oxide anion and resulting in the excitation of electrons. In order to quantify the exact amount of energy transferred to the surface, the molecules final translational and rotational energy has to be known in addition to the final vibrational energy. Time-offlight experiments on initially ...
... the nitric oxide anion and resulting in the excitation of electrons. In order to quantify the exact amount of energy transferred to the surface, the molecules final translational and rotational energy has to be known in addition to the final vibrational energy. Time-offlight experiments on initially ...
- Career Point Kota
... the P4 molecule where the angle are only 60 & also they have low M.P.” (b) Electron gain enthalpy of halogen are largely negativity it is due to the fact that they have high effective nuclear charge & smallest size among period. Although they contain 7eΘ in valence shell & required one electron to a ...
... the P4 molecule where the angle are only 60 & also they have low M.P.” (b) Electron gain enthalpy of halogen are largely negativity it is due to the fact that they have high effective nuclear charge & smallest size among period. Although they contain 7eΘ in valence shell & required one electron to a ...
Chemistry
... You might wonder why the study of chemistry is so important if you can’t use it to turn iron into gold or to develop a potion that will make you immortal. Why didn’t chemistry die when scientists like Boyle and Lavoisier proved alchemy was nothing but a hoax? Although we can’t use chemistry to make ...
... You might wonder why the study of chemistry is so important if you can’t use it to turn iron into gold or to develop a potion that will make you immortal. Why didn’t chemistry die when scientists like Boyle and Lavoisier proved alchemy was nothing but a hoax? Although we can’t use chemistry to make ...
Atoms and Molecules
... Each element is composed of atoms – which are incredibly small. All atoms of a given element are identical to one another in mass and other properties, and different from all other atoms. That atoms were indivisible, and were not created or destroyed in chemical reactions. When atoms of different el ...
... Each element is composed of atoms – which are incredibly small. All atoms of a given element are identical to one another in mass and other properties, and different from all other atoms. That atoms were indivisible, and were not created or destroyed in chemical reactions. When atoms of different el ...
Η - Knockhardy
... To aid balancing the equation, remember that you get one carbon dioxide molecule for every carbon atom in the original molecule and a water molecule for every two hydrogen atoms. When you have done this, go back and balance the oxygen. ...
... To aid balancing the equation, remember that you get one carbon dioxide molecule for every carbon atom in the original molecule and a water molecule for every two hydrogen atoms. When you have done this, go back and balance the oxygen. ...
Unit - II Electrochemistry
... At equilibrium, the potential difference becomes a constant value which is known as the electrode potential of the metal. Thus the tendency of the electrode to lose electrons is called Oxidation potential and tendency of an electrode to gain electrons is called reduction potential. Single electrode ...
... At equilibrium, the potential difference becomes a constant value which is known as the electrode potential of the metal. Thus the tendency of the electrode to lose electrons is called Oxidation potential and tendency of an electrode to gain electrons is called reduction potential. Single electrode ...
2011-2012 ACAD REVIEW SHEET Chapter 2
... (ANS: both the filtration apparatus and the distillation apparatus is shown in Figure 2-23 on page 78. An important part to include in filtration is that it separates by particle size and with respect to distillation is that it separates by boiling point differences.) ...
... (ANS: both the filtration apparatus and the distillation apparatus is shown in Figure 2-23 on page 78. An important part to include in filtration is that it separates by particle size and with respect to distillation is that it separates by boiling point differences.) ...
10934_2017_374_MOESM1_ESM
... The BET surface areas were observed as 838 m2/g for 0h, 789 m2/g for 4 days and 784 m2/g for 14 days after hydrothermal test. This result of hydrothermal test was slightly different than that of the smaller scale synthesis(Figure 4). We hypothesized that hot filtration may have caused higher surface ...
... The BET surface areas were observed as 838 m2/g for 0h, 789 m2/g for 4 days and 784 m2/g for 14 days after hydrothermal test. This result of hydrothermal test was slightly different than that of the smaller scale synthesis(Figure 4). We hypothesized that hot filtration may have caused higher surface ...
Chemical bonding
... 22) How many lone pairs of electrons are present in a molecule of SF4? 23) What is hybridization? 24) Write the shape and bond angle of sp hybrid orbitals? 25) Give an example of a molecule having sp hybridization? 26) What is the percentage of s character in sp hybridization? 27) What is the shape ...
... 22) How many lone pairs of electrons are present in a molecule of SF4? 23) What is hybridization? 24) Write the shape and bond angle of sp hybrid orbitals? 25) Give an example of a molecule having sp hybridization? 26) What is the percentage of s character in sp hybridization? 27) What is the shape ...
Stoichiometry - VernonScienceLSA
... In these types of calculations, the reactant that it present in lesser amount is called the LIMITING REACTANT. Since the limiting reactant gets completely used up first, it sets the limit on the amount of product that can be formed and the amount of the excess reactant used in the reaction. LIMITING ...
... In these types of calculations, the reactant that it present in lesser amount is called the LIMITING REACTANT. Since the limiting reactant gets completely used up first, it sets the limit on the amount of product that can be formed and the amount of the excess reactant used in the reaction. LIMITING ...
Calculations on the equations reaction
... 1. An element has serial number 11 define: а) charge of nucleus atom b) number of electrons c) number of neutrons and protons. Write electronic formula of element. What valences this element can have in compounds? Write the formula of highest oxide of this element. 2. An element has serial number 19 ...
... 1. An element has serial number 11 define: а) charge of nucleus atom b) number of electrons c) number of neutrons and protons. Write electronic formula of element. What valences this element can have in compounds? Write the formula of highest oxide of this element. 2. An element has serial number 19 ...