Answers to 2017 Chemistry Exam Review Compounds and
... 75. Temperature relates to the kinetic energy or speed of molecules. Pressure relates to how often and how hard the molecules hit each other or other things. 76. Atmospheric pressure depends on the weight of air above = about 15 psi = 1 atm at sea level. It decreases altitude increases b/c of less w ...
... 75. Temperature relates to the kinetic energy or speed of molecules. Pressure relates to how often and how hard the molecules hit each other or other things. 76. Atmospheric pressure depends on the weight of air above = about 15 psi = 1 atm at sea level. It decreases altitude increases b/c of less w ...
7.P.2A.1 GT Notes
... 7.P.2A.1 Develop and use simple atomic models to illustrate the components of elements (including the relative position and charge of protons, neutrons, and electrons). 1. Name the subatomic particles atoms are made of that affect the properties of an atom. ...
... 7.P.2A.1 Develop and use simple atomic models to illustrate the components of elements (including the relative position and charge of protons, neutrons, and electrons). 1. Name the subatomic particles atoms are made of that affect the properties of an atom. ...
Chapter 4 Quiz ____ 1. The Greek philosopher Democritus coined
... ____ 2. Which of the following is NOT part of John Dalton’s atomic theory? a. All elements are composed of atoms. b. All atoms of the same element have the same mass. c. Atoms contain subatomic particles. d. A compound contains atoms of more than one element. ____ 3. Rutherford’s gold foil experimen ...
... ____ 2. Which of the following is NOT part of John Dalton’s atomic theory? a. All elements are composed of atoms. b. All atoms of the same element have the same mass. c. Atoms contain subatomic particles. d. A compound contains atoms of more than one element. ____ 3. Rutherford’s gold foil experimen ...
Chapter 2. The Chemical Context of Life
... Pair of electrons not shared equally by 2 atoms Water = O + H oxygen has stronger “attraction” for the shared electrons than hydrogen oxygen has higher ...
... Pair of electrons not shared equally by 2 atoms Water = O + H oxygen has stronger “attraction” for the shared electrons than hydrogen oxygen has higher ...
Chapter 7 Periodic Properties of the Elements
... • Elements gain or lose electrons to acquire the electron configuration of the noble gases. Periodic Properties of the Elements ...
... • Elements gain or lose electrons to acquire the electron configuration of the noble gases. Periodic Properties of the Elements ...
atoms
... Organized by increasing atomic number Periods (rows) are labeled 1-7 (# of electrons) 18 Columns of elements contains a group (family) of elements that have similar properties. (Properties Repeat -Periodic Law) The number of an “A” group element matches the number of valence electrons for an element ...
... Organized by increasing atomic number Periods (rows) are labeled 1-7 (# of electrons) 18 Columns of elements contains a group (family) of elements that have similar properties. (Properties Repeat -Periodic Law) The number of an “A” group element matches the number of valence electrons for an element ...
CH 5-7 Chapter 5-7 review wkey
... 20. How many milliliters of 0.123 M NaOH solution contain 25.0 g of NaOH (molar mass = 40.00 g/mol)? a) 5.08 mL d) 625 mL b) 50.8 mL e) 5080 mL c) 508 mL 21. If you need 1.00 L of 0.125 M H2SO4, how would you prepare this solution? a) Add 950. mL of water to 50.0 mL of 3.00 M H2SO4. b) Add 500. mL o ...
... 20. How many milliliters of 0.123 M NaOH solution contain 25.0 g of NaOH (molar mass = 40.00 g/mol)? a) 5.08 mL d) 625 mL b) 50.8 mL e) 5080 mL c) 508 mL 21. If you need 1.00 L of 0.125 M H2SO4, how would you prepare this solution? a) Add 950. mL of water to 50.0 mL of 3.00 M H2SO4. b) Add 500. mL o ...
Quantitative periodic table – dominoes
... a complete ordered set of cards. Differentiation: the cards generally increase in difficulty from the start to the end. In particular, the last 5 cards require more mathematical processing. When attempting this activity, students should have already studied atomic structure (including protons, elect ...
... a complete ordered set of cards. Differentiation: the cards generally increase in difficulty from the start to the end. In particular, the last 5 cards require more mathematical processing. When attempting this activity, students should have already studied atomic structure (including protons, elect ...
Atoms, Elements, Compounds, and Mixtures
... be divided and still maintain its years, scientists have characteristics. designed many different models for this structure. Atoms are the building blocks of the universe. There are 92 Each one was the best different kinds of atoms that model at the time, but as occur naturally, although more ne ...
... be divided and still maintain its years, scientists have characteristics. designed many different models for this structure. Atoms are the building blocks of the universe. There are 92 Each one was the best different kinds of atoms that model at the time, but as occur naturally, although more ne ...
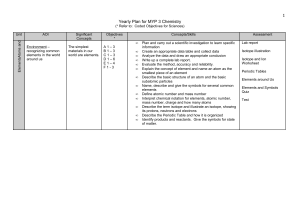
Yearly Plan for MYP 1 Science
... Explain the concept of element and name an atom as the smallest piece of an element Describe the basic structure of an atom and the basic subatomic particles Name, describe and give the symbols for several common elements Define atomic number and mass number Interpret chemical notation for elements, ...
... Explain the concept of element and name an atom as the smallest piece of an element Describe the basic structure of an atom and the basic subatomic particles Name, describe and give the symbols for several common elements Define atomic number and mass number Interpret chemical notation for elements, ...
Section 3.3 Day 1
... of Thomson’s plum pudding model with a nuclear model of the atom. Rutherford suggested that electrons are like planets orbiting the sun and revolve around the nucleus in circular or elliptical orbits. Because Rutherford’s model could not explain why electrons did not crash into the nucleus, it was ...
... of Thomson’s plum pudding model with a nuclear model of the atom. Rutherford suggested that electrons are like planets orbiting the sun and revolve around the nucleus in circular or elliptical orbits. Because Rutherford’s model could not explain why electrons did not crash into the nucleus, it was ...
Bohr awarded Nobel prize for physics in 1922
... increased in intensity of reactions c. Conclusion >> Slow Neutrons are better at causing fissions d. This is because slow neutrons are unrepelled by the nucleus and spend greater time in vicinity of nucleus >> more chance of being captured 3. Lise Meitner and Frisch Strassman Identify Fission a. Mei ...
... increased in intensity of reactions c. Conclusion >> Slow Neutrons are better at causing fissions d. This is because slow neutrons are unrepelled by the nucleus and spend greater time in vicinity of nucleus >> more chance of being captured 3. Lise Meitner and Frisch Strassman Identify Fission a. Mei ...
CHAPTER 8: Atomic Physics
... Electrons have anti-aligned (ms = +½ and ms = −½) spins as being paired , then the cancel, total spin becomes an integer (0), i.e. the whole particle becomes a boson, composed of fermions (which are subject to the Pauli exclusion principle, nuclear spin cancel also, happens at there are two proton ...
... Electrons have anti-aligned (ms = +½ and ms = −½) spins as being paired , then the cancel, total spin becomes an integer (0), i.e. the whole particle becomes a boson, composed of fermions (which are subject to the Pauli exclusion principle, nuclear spin cancel also, happens at there are two proton ...
CHEM Notes Unit 4 History of atomic theory Monday Oct 7 Greek
... there could not be a location where there is nothing. Both sets of ideas were known and studied for the next 2000 years. HOWEVER, there weren’t many schools, not many people could even read, and most people who studied matter were ALCHEMISTS – studied matter more as if it was magic – they weren’t sc ...
... there could not be a location where there is nothing. Both sets of ideas were known and studied for the next 2000 years. HOWEVER, there weren’t many schools, not many people could even read, and most people who studied matter were ALCHEMISTS – studied matter more as if it was magic – they weren’t sc ...
Atomic masses are weighted averages.
... chemically combine in simple whole-number ratios to form compounds. This is known as the Law of Definite Proportions – very important. ...
... chemically combine in simple whole-number ratios to form compounds. This is known as the Law of Definite Proportions – very important. ...
Structure of Atoms
... 99% of carbon atoms have 6 neutrons (12C). Most of the remaining 1% of carbon atoms have 7 neutrons (13C) while the rarest carbon isotope, with 8 neutrons, is 14C. Both 12C and 13C are stable isotopes while 14C is radioactive When 14C decays, one of its neutrons is converted to a proton and an ...
... 99% of carbon atoms have 6 neutrons (12C). Most of the remaining 1% of carbon atoms have 7 neutrons (13C) while the rarest carbon isotope, with 8 neutrons, is 14C. Both 12C and 13C are stable isotopes while 14C is radioactive When 14C decays, one of its neutrons is converted to a proton and an ...
honors chem 6 day review packet
... Metals _____________ electrons. They form __________________. Nonmetals ______________________ electrons. They form __________. Trends Atomic radius (size) ionization energy (energy to remove an electron) Electronegativity (ability to gain an electron) Reactivity for metal (metallic character) React ...
... Metals _____________ electrons. They form __________________. Nonmetals ______________________ electrons. They form __________. Trends Atomic radius (size) ionization energy (energy to remove an electron) Electronegativity (ability to gain an electron) Reactivity for metal (metallic character) React ...
Key concepts of chemistry from high school chemistry
... The atomic mass unit scale is devised because of the extremely small size and low mass of atoms. In a 12.0000000 g sample of pure carbon-‐12, there are precisely 6.02214179x1023 ato ...
... The atomic mass unit scale is devised because of the extremely small size and low mass of atoms. In a 12.0000000 g sample of pure carbon-‐12, there are precisely 6.02214179x1023 ato ...
Key Concept 1: An atom is the smallest unit of an element that
... Key Concept 7: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. ...
... Key Concept 7: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. ...
key concepts of matter
... protons located in its nucleus. Key Concept 2: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. Key Concept 3: Electrons located in the outermost shell of the electron cloud are ...
... protons located in its nucleus. Key Concept 2: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. Key Concept 3: Electrons located in the outermost shell of the electron cloud are ...
Atoms and Elements
... • Electrons always fill up the lower energy levels first, just like cars in a parking garage fill in the spaces closest to the ground floor, then spread farther up as the spots get filled. ...
... • Electrons always fill up the lower energy levels first, just like cars in a parking garage fill in the spaces closest to the ground floor, then spread farther up as the spots get filled. ...
7th Science - Carterville CUSD #5
... 13. Atoms of the SAME ELEMENT always have the SAME NUMBER OF ________________. The two ways that atoms of the SAME ELEMENT can be different are if the number of _______________________ changes (then it is called an ___________________) or the number of _________________________ changes (this is call ...
... 13. Atoms of the SAME ELEMENT always have the SAME NUMBER OF ________________. The two ways that atoms of the SAME ELEMENT can be different are if the number of _______________________ changes (then it is called an ___________________) or the number of _________________________ changes (this is call ...
1 km = 1 000 m 1 m = 100 cm 1 cm = 10 mm 1 m = 1 000 mm
... Niels Bohr quickly seized upon this used it to propose a quantized description of the atom. 1. Bohr proposed that while circling the nucleus of the atom, electrons could only occupy certain discrete orbits, that is to say energy levels. Bohr used Max Planck's equations describing quanta of radiation ...
... Niels Bohr quickly seized upon this used it to propose a quantized description of the atom. 1. Bohr proposed that while circling the nucleus of the atom, electrons could only occupy certain discrete orbits, that is to say energy levels. Bohr used Max Planck's equations describing quanta of radiation ...
Unit 2 – Atomic Theory - H
... Element Symbol with mass number and atomic number Can also be the element name dash mass number Mass Number ...
... Element Symbol with mass number and atomic number Can also be the element name dash mass number Mass Number ...