Atomic Structure Tick Sheet
... NUMBERS of positive protons and negative electrons so the charges cancel. I know that all atoms of the same element have the SAME number of protons. I know that atoms of DIFFERENT elements have DIFFERENT numbers of protons. I know that the ATOMIC NUMBER of an atom is the BOTTOM NUMBER next to the sy ...
... NUMBERS of positive protons and negative electrons so the charges cancel. I know that all atoms of the same element have the SAME number of protons. I know that atoms of DIFFERENT elements have DIFFERENT numbers of protons. I know that the ATOMIC NUMBER of an atom is the BOTTOM NUMBER next to the sy ...
Click here have a readable handout.
... Ernest Rutherford conducted a famous experiment called the gold foil experiment. He took a thin sheet of gold foil. He used special equipment to shoot alpha particles (positively charged particles) at the gold foil. Most particles passed straight through the foil like the foil was not there. Some pa ...
... Ernest Rutherford conducted a famous experiment called the gold foil experiment. He took a thin sheet of gold foil. He used special equipment to shoot alpha particles (positively charged particles) at the gold foil. Most particles passed straight through the foil like the foil was not there. Some pa ...
weighted average - Effingham County Schools
... definition of an element as a substance that cannot be further broken down by ordinary chemical means. •It was also clear that elements combine to form compounds that have different physical and chemical properties than those of the elements that form them. Na + Cl → NaCl ...
... definition of an element as a substance that cannot be further broken down by ordinary chemical means. •It was also clear that elements combine to form compounds that have different physical and chemical properties than those of the elements that form them. Na + Cl → NaCl ...
Understanding the Properties of Elements – Chapter 5
... 2. Name the non-metal ion second. The end of its name will change to “-ide”. 3. If the proportion of one element to the other is not 1:1, a subscript is required to indicate the proportion of the element that is more abundant. e.g. For 1 Magnesium atom, there are 2 chlorine atoms: MgCl2. ...
... 2. Name the non-metal ion second. The end of its name will change to “-ide”. 3. If the proportion of one element to the other is not 1:1, a subscript is required to indicate the proportion of the element that is more abundant. e.g. For 1 Magnesium atom, there are 2 chlorine atoms: MgCl2. ...
Atomic theory gallery walk
... Ernest Rutherford conducted a famous experiment called the gold foil experiment. He took a thin sheet of gold foil. He used special equipment to shoot alpha particles (positively charged particles) at the gold foil. Most particles passed straight through the foil like the foil was not there. Some pa ...
... Ernest Rutherford conducted a famous experiment called the gold foil experiment. He took a thin sheet of gold foil. He used special equipment to shoot alpha particles (positively charged particles) at the gold foil. Most particles passed straight through the foil like the foil was not there. Some pa ...
Stoichiometry
... if we were able to count atoms at the rate of 10 million per second, it would take about 2 billion years to count an Avogadro’s number of atoms ...
... if we were able to count atoms at the rate of 10 million per second, it would take about 2 billion years to count an Avogadro’s number of atoms ...
Carbohydrates
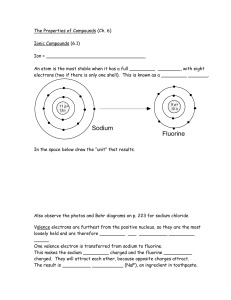
... these electrons and completes this octet, or set of eight, by interacting with other atoms. Note: The exception to this is that hydrogen and helium want 2 electrons in the outer shell. • Atoms form chemical bonds in an attempt to fill their outer shells. If an atom already has a full outer shell, it ...
... these electrons and completes this octet, or set of eight, by interacting with other atoms. Note: The exception to this is that hydrogen and helium want 2 electrons in the outer shell. • Atoms form chemical bonds in an attempt to fill their outer shells. If an atom already has a full outer shell, it ...
Biochem
... these electrons and completes this octet, or set of eight, by interacting with other atoms. Note: The exception to this is that hydrogen and helium want 2 electrons in the outer shell. • Atoms form chemical bonds in an attempt to fill their outer shells. If an atom already has a full outer shell, it ...
... these electrons and completes this octet, or set of eight, by interacting with other atoms. Note: The exception to this is that hydrogen and helium want 2 electrons in the outer shell. • Atoms form chemical bonds in an attempt to fill their outer shells. If an atom already has a full outer shell, it ...
Atomic Theory - hrsbstaff.ednet.ns.ca
... energy levels are quantized. In contrast, if any energy level were allowed, the emission spectrum would be continuous. This means, electrons can only be at certain energy levels around a nucleus. Convergence is a mathematical term for a series of numbers that gradually decrease, but never quite get ...
... energy levels are quantized. In contrast, if any energy level were allowed, the emission spectrum would be continuous. This means, electrons can only be at certain energy levels around a nucleus. Convergence is a mathematical term for a series of numbers that gradually decrease, but never quite get ...
The History of Atomic Theory Lexile 1260L 8.5A: Atoms
... each atom. He then calculated they had a much smaller mass than that of other particles, protons and neutrons. ...
... each atom. He then calculated they had a much smaller mass than that of other particles, protons and neutrons. ...
Summer - Honors Chemistry
... B Elements, Compounds, & Mixtures: There are about 114 elements known; of these 90 are naturally occurring on earth. Each element has a one or two letter symbol with one capital letter (e.g. B and Br). Elements are made from only one kind of atom (which all share the same atomic number and elemental ...
... B Elements, Compounds, & Mixtures: There are about 114 elements known; of these 90 are naturally occurring on earth. Each element has a one or two letter symbol with one capital letter (e.g. B and Br). Elements are made from only one kind of atom (which all share the same atomic number and elemental ...
Unit 01 Qual Chem
... Physical Change = a change that does not alter the identity of a substance (shape, size, ...
... Physical Change = a change that does not alter the identity of a substance (shape, size, ...
Syracuse University
... current Accommodation Authorization Letter from ODS to the instructor and review those accommodations with the instructor. Accommodations, such as exam administration, are not provided retroactively; therefore, planning for accommodations as early as possible is necessary. Religious Policies: SU’s r ...
... current Accommodation Authorization Letter from ODS to the instructor and review those accommodations with the instructor. Accommodations, such as exam administration, are not provided retroactively; therefore, planning for accommodations as early as possible is necessary. Religious Policies: SU’s r ...
History of the atoms - Brookwood High School
... 1. Matter is composed of air, fire, water, and Earth ...
... 1. Matter is composed of air, fire, water, and Earth ...
Atom
... What we know now of Dalton’s Atomic Theory 1. All elements are composed of tiny indivisible particles called atoms. Atoms are not indivisible – they are made of subatomic particles 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element ...
... What we know now of Dalton’s Atomic Theory 1. All elements are composed of tiny indivisible particles called atoms. Atoms are not indivisible – they are made of subatomic particles 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element ...
Introduction to Atomic Structure - California K
... CaMSP Science Matters Summer Institute 2007 Petra van Koppen Page 1 of 6 ...
... CaMSP Science Matters Summer Institute 2007 Petra van Koppen Page 1 of 6 ...
10C The Periodic Table
... table charts, and information about them, in your local library or on the Internet. A periodic table holds an amazing amount of information. In this skill sheet, you will use a periodic table to identify information about specific elements, make calculations, and make predictions. Read: ...
... table charts, and information about them, in your local library or on the Internet. A periodic table holds an amazing amount of information. In this skill sheet, you will use a periodic table to identify information about specific elements, make calculations, and make predictions. Read: ...
Chapter 11: The Atomic Nature of Matter
... • Uncharged particles in the neutron, with mass ~ that of proton. • The # of neutrons need not match # of protons in atom, eg. H typically has 1 proton and 0 neutrons, but some H atoms may have 1 neutron, but always 1 proton, (called “heavy hydrogen”) • Isotopes = atoms of same element that contain ...
... • Uncharged particles in the neutron, with mass ~ that of proton. • The # of neutrons need not match # of protons in atom, eg. H typically has 1 proton and 0 neutrons, but some H atoms may have 1 neutron, but always 1 proton, (called “heavy hydrogen”) • Isotopes = atoms of same element that contain ...
Chapter 1
... That model is simplicity itself. All matter is composed of atoms, which themselves form aggregates called molecules. An atom contains a positive nucleus very much smaller than the full atom. A nucleus with atomic mass A contains Z protons and A − Z neutrons. The neutral atom has, as well, Z electron ...
... That model is simplicity itself. All matter is composed of atoms, which themselves form aggregates called molecules. An atom contains a positive nucleus very much smaller than the full atom. A nucleus with atomic mass A contains Z protons and A − Z neutrons. The neutral atom has, as well, Z electron ...
Atomic Theory and Bonding
... • Electrons appear in shells in a very predictable manner. • There is a maximum of 2 electrons in the first shell, 8 in the 2nd shell, and 8 in the 3rd shell. The period # = # of shells in the atom. Except for the transition elements, the last digit of the group # = # of electrons in the valence ...
... • Electrons appear in shells in a very predictable manner. • There is a maximum of 2 electrons in the first shell, 8 in the 2nd shell, and 8 in the 3rd shell. The period # = # of shells in the atom. Except for the transition elements, the last digit of the group # = # of electrons in the valence ...
Atomic Theory and Bonding
... • Electrons appear in shells in a very predictable manner. • There is a maximum of 2 electrons in the first shell, 8 in the 2nd shell, and 8 in the 3rd shell. The period # = # of shells in the atom. Except for the transition elements, the last digit of the group # = # of electrons in the valence ...
... • Electrons appear in shells in a very predictable manner. • There is a maximum of 2 electrons in the first shell, 8 in the 2nd shell, and 8 in the 3rd shell. The period # = # of shells in the atom. Except for the transition elements, the last digit of the group # = # of electrons in the valence ...