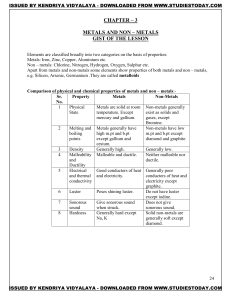
METALS AND NON – METALS Concepts
... + Cl2 2NaCl Metals react with hydrogen to form metal hydride This reaction takes place only for most reactive metals. 2Na(s) + H2(g) 2NaH(s) ...
... + Cl2 2NaCl Metals react with hydrogen to form metal hydride This reaction takes place only for most reactive metals. 2Na(s) + H2(g) 2NaH(s) ...
Unit 5: Electrochemistry
... 2. Using Standard Reduction Potentials to predict Spontaneous Reactions The larger the Potential (in V), the more readily reduced the substance is. The smaller the Potential, the more readily oxidized it is. So, to choose which is oxidized and which is reduced, look to the table and the one with th ...
... 2. Using Standard Reduction Potentials to predict Spontaneous Reactions The larger the Potential (in V), the more readily reduced the substance is. The smaller the Potential, the more readily oxidized it is. So, to choose which is oxidized and which is reduced, look to the table and the one with th ...
Unit 3: Chemistry. Introduction to Atoms. Atomic mass
... Fun Fact: If it were possible to have a nucleus the volume of an average grape, that nucleus would have a mass greater than 9 MILLION TONS! ...
... Fun Fact: If it were possible to have a nucleus the volume of an average grape, that nucleus would have a mass greater than 9 MILLION TONS! ...
THERMAL ENERGY
... Similarities of behavior in the periodic table are due to the similarities in the electron arrangement of the atoms. This arrangement is called electron configuration. The modern model of the atom describes the electron cloud consisting of separate energy levels, each containing a fixed number o ...
... Similarities of behavior in the periodic table are due to the similarities in the electron arrangement of the atoms. This arrangement is called electron configuration. The modern model of the atom describes the electron cloud consisting of separate energy levels, each containing a fixed number o ...
Atomic Structure Powerpoint
... Bohr was incorrect in assuming that electrons moved like planets in a solar system. The Electron Cloud model shows the MOST LIKELY location of electrons in an atom!! (these are not precise since it’s all based on probability!!) ...
... Bohr was incorrect in assuming that electrons moved like planets in a solar system. The Electron Cloud model shows the MOST LIKELY location of electrons in an atom!! (these are not precise since it’s all based on probability!!) ...
subshells
... Indicate which statement is not true in the ordering of the periodic table ? a) The electrons tend to occupy the lowest energy levels available to them b) No two electrons in an atom can have the same set of quantum numbers (n , l , me , ms) c) Electrons with higher l values go earlier into unfilled ...
... Indicate which statement is not true in the ordering of the periodic table ? a) The electrons tend to occupy the lowest energy levels available to them b) No two electrons in an atom can have the same set of quantum numbers (n , l , me , ms) c) Electrons with higher l values go earlier into unfilled ...
Nature of Atoms Atomic Structure Atomic number Atomic mass
... Molecules are groups of atoms held together in a stable association Compounds are molecules containing yp of element more than one type Atoms are held together in molecules or compounds by chemical bonds ...
... Molecules are groups of atoms held together in a stable association Compounds are molecules containing yp of element more than one type Atoms are held together in molecules or compounds by chemical bonds ...
Atoms, Elements, and Compounds
... Marie Curie’s idea was revolutionary because atoms were still believed to be tiny, featureless particles. She decided to test every known element to see if any others would, like uranium, improve the air’s ability to conduct electricity. She found that the element thorium had this property. Pierre C ...
... Marie Curie’s idea was revolutionary because atoms were still believed to be tiny, featureless particles. She decided to test every known element to see if any others would, like uranium, improve the air’s ability to conduct electricity. She found that the element thorium had this property. Pierre C ...
Chapter 3B Modern Atomic Theory CHAPTER OUTLINE
... ionization energy and metallic character. q These properties are commonly known as periodic properties and increase or decrease across a period or group, and are repeated in each successive period or group. ...
... ionization energy and metallic character. q These properties are commonly known as periodic properties and increase or decrease across a period or group, and are repeated in each successive period or group. ...
04 Atom notes
... using _______________________________________________________________ _____________________________________________________________________ How was John Dalton able to study atoms even though he was unable to see them directly? What evidence did he use to form his theory? ___________________________ ...
... using _______________________________________________________________ _____________________________________________________________________ How was John Dalton able to study atoms even though he was unable to see them directly? What evidence did he use to form his theory? ___________________________ ...
Oxygen-16 Charge of 0 Chlorine-36 Charge of -1 Sulfur-33 Charge -2
... Name ______________________________________ Date ________________ Period ___________________ Draw the atomic structure here Atomic Number ________________ Number of Protons ______________ Number of Neutrons _____________ ...
... Name ______________________________________ Date ________________ Period ___________________ Draw the atomic structure here Atomic Number ________________ Number of Protons ______________ Number of Neutrons _____________ ...
Atomic Structure - davis.k12.ut.us
... Molecules - a group of atoms. 7. Thompson’s experiment – cathode ray tube found electron negative His atomic model – Plum pudding model 8. Rutherford’s experiment – gold foil experiment His atomic model – planatary model 9. Atomic number - the number of protons ...
... Molecules - a group of atoms. 7. Thompson’s experiment – cathode ray tube found electron negative His atomic model – Plum pudding model 8. Rutherford’s experiment – gold foil experiment His atomic model – planatary model 9. Atomic number - the number of protons ...
Unit 9 The p-Block Elements
... structure, every carbon atom forms four covalent bonds by sharing electrons with each of its four nearest neighbours. Silicon and germanium crystallize in the same giant covalent structure as diamond. In graphite, the carbon atoms are arranged in flat, parallel layers. Each layer contains millions o ...
... structure, every carbon atom forms four covalent bonds by sharing electrons with each of its four nearest neighbours. Silicon and germanium crystallize in the same giant covalent structure as diamond. In graphite, the carbon atoms are arranged in flat, parallel layers. Each layer contains millions o ...
Chapter 07 and 08 Chemical Bonding and Molecular
... • Made of 2 or more elements in a definite proportion by mass • Physically and chemically different from the elements that make up the compound • All elements (except Noble gases) react to gain a stable octet. (duet-for H through B) • Compounds form to gain a stable valence shell which is LOWER IN E ...
... • Made of 2 or more elements in a definite proportion by mass • Physically and chemically different from the elements that make up the compound • All elements (except Noble gases) react to gain a stable octet. (duet-for H through B) • Compounds form to gain a stable valence shell which is LOWER IN E ...
Average Atomic Mass notes
... – We have different isotopes of the same atom • They are the same type of atom but they have a different mass because they have a different number of neutrons ...
... – We have different isotopes of the same atom • They are the same type of atom but they have a different mass because they have a different number of neutrons ...
Ch. 02 - HCC Learning Web
... • Valence electrons are those in the outermost shell, or valence shell • The chemical behavior of an atom is mostly determined by the valence electrons • Elements with a full valence shell are ...
... • Valence electrons are those in the outermost shell, or valence shell • The chemical behavior of an atom is mostly determined by the valence electrons • Elements with a full valence shell are ...
About 440 B.C. Empedocles stated that all matter was composed of
... atom. Since alpha particles are relatively high in mass, the extent of the deflections, remember some actually bounced back, indicated to Rutherford that the nucleus is relatively very heavy and dense. Since most of the alpha particles passed through the thousand or so gold atoms without any apparen ...
... atom. Since alpha particles are relatively high in mass, the extent of the deflections, remember some actually bounced back, indicated to Rutherford that the nucleus is relatively very heavy and dense. Since most of the alpha particles passed through the thousand or so gold atoms without any apparen ...
- Dr.Divan Fard
... The number of subshells in a given shell is equal to the shell number. For example, shell number 3 has 3 subshells. Within each subshell, electrons are further grouped into orbitals, regions of space within an atom where the specific electrons are more likely to be found. There are different number ...
... The number of subshells in a given shell is equal to the shell number. For example, shell number 3 has 3 subshells. Within each subshell, electrons are further grouped into orbitals, regions of space within an atom where the specific electrons are more likely to be found. There are different number ...
Honors Chemistry
... general trend: As size decreases, solubility increases. Some helpful notes on writing phases in chemical reactions: 1. Metals are solids (except ...
... general trend: As size decreases, solubility increases. Some helpful notes on writing phases in chemical reactions: 1. Metals are solids (except ...
Chapter 4 - H - Regional School District 17
... The network of blood vessels in your body is like the network of streets and highways in a large city. How are the two networks similar? Both networks are used to transport objects from one location to another. The comparison is an example of an analogy. An analogy uses a similarity to compare two o ...
... The network of blood vessels in your body is like the network of streets and highways in a large city. How are the two networks similar? Both networks are used to transport objects from one location to another. The comparison is an example of an analogy. An analogy uses a similarity to compare two o ...
The Chemistry of Life
... The electrons that are available to form bonds are called valence electrons. The main types of chemical bonds are ionic bonds and covalent bonds. ...
... The electrons that are available to form bonds are called valence electrons. The main types of chemical bonds are ionic bonds and covalent bonds. ...
Organometallic Chemistry at the Magnesium− Tris (8
... O(1s) are not so easily interpreted in terms of a model in which the quinolinate ligands of Alq3 undergo simple reduction: These calculations show that, even though the LUMO is maximized on the pyridyl ring, some increase in negative charge also accrues to the phenolic ring of the quinolinate ligand ...
... O(1s) are not so easily interpreted in terms of a model in which the quinolinate ligands of Alq3 undergo simple reduction: These calculations show that, even though the LUMO is maximized on the pyridyl ring, some increase in negative charge also accrues to the phenolic ring of the quinolinate ligand ...
GHW - Louisiana Tech University
... The gram mole is the grams of any chemical substance using the value atomic mass obtained from the periodic table. E.g. for carbon gram mole is 12.01 grams of carbon since its atomic mass is 12.01 amu in the periodic table. if you take atomic mass in grams the number of atoms is simply 6.022 x 10 23 ...
... The gram mole is the grams of any chemical substance using the value atomic mass obtained from the periodic table. E.g. for carbon gram mole is 12.01 grams of carbon since its atomic mass is 12.01 amu in the periodic table. if you take atomic mass in grams the number of atoms is simply 6.022 x 10 23 ...
The Flow of Energy: Heat
... effective nuclear charge. • Within each group (vertical column), the atomic radius tends to increase with the period number. ...
... effective nuclear charge. • Within each group (vertical column), the atomic radius tends to increase with the period number. ...























