Introduction to Chemistry
... Ionic- Two elements bond by transferring electrons to create ions that attract together (+ is attracted to - after an electron is transferred) ...
... Ionic- Two elements bond by transferring electrons to create ions that attract together (+ is attracted to - after an electron is transferred) ...
Chapter 4 Homework 4 File
... There are five naturally occurring isotopes of the element zinc. The relative abundance and mass of each are as follows: ...
... There are five naturally occurring isotopes of the element zinc. The relative abundance and mass of each are as follows: ...
AP Review to Share - Wappingers Central School District
... If e- are still left at this point, assign them to the central atom. If the central atom is from the third or a higher period, it can accommodate more than four pairs of electrons. If the central atom is not yet surrounded by ...
... If e- are still left at this point, assign them to the central atom. If the central atom is from the third or a higher period, it can accommodate more than four pairs of electrons. If the central atom is not yet surrounded by ...
nuclear properties
... many possibilities for each element. Different isotopes of same element have different numbers of neutrons N = neutron number ex: A nucleus with 6 protons and 6 neutrons is different from a nucleus with 6 protons and 7 neutrons They are different isotopes of carbon differ in # of neutrons ...
... many possibilities for each element. Different isotopes of same element have different numbers of neutrons N = neutron number ex: A nucleus with 6 protons and 6 neutrons is different from a nucleus with 6 protons and 7 neutrons They are different isotopes of carbon differ in # of neutrons ...
50 Forgotten Facts
... 39) In Le Chatelier’s Principle, if a system is at equilibrium, if something is added, then the equilibrium will shift away from the side it is on. If something is removed, then the equilibrium will shift towards that side. After the shift, whatever is being shifted towards will increase in concentr ...
... 39) In Le Chatelier’s Principle, if a system is at equilibrium, if something is added, then the equilibrium will shift away from the side it is on. If something is removed, then the equilibrium will shift towards that side. After the shift, whatever is being shifted towards will increase in concentr ...
Chapter 1 Introduction: Matter and Measurement
... random motion unless constrained. Use Ar atom as an example. Draw diagrams of s, l, and g Ar and use them to show how this theory explains all of the observations in chart above describing properties of states of matter ...
... random motion unless constrained. Use Ar atom as an example. Draw diagrams of s, l, and g Ar and use them to show how this theory explains all of the observations in chart above describing properties of states of matter ...
All you need to know about Additional Science
... If we have a solution containing 100 g of sodium hydroxide, how much chlorine gas should we pass through the solution to make bleach? Too much, and some chlorine will be wasted, too little and not all of the sodium hydroxide will react. ...
... If we have a solution containing 100 g of sodium hydroxide, how much chlorine gas should we pass through the solution to make bleach? Too much, and some chlorine will be wasted, too little and not all of the sodium hydroxide will react. ...
atoms.
... • Aluminum containers are lightweight because of the properties of the Al atoms that make them up. • Nanotechnology (making products that are atom-size) is being used to make microsubmarines which will eventually be able to travel in our bodies to detect health problems. ...
... • Aluminum containers are lightweight because of the properties of the Al atoms that make them up. • Nanotechnology (making products that are atom-size) is being used to make microsubmarines which will eventually be able to travel in our bodies to detect health problems. ...
Atoms
... occurring isotopes with mass numbers of 121 and 123. The relative abundance and atomic masses are 57.12 % for mass = 120.90 amu, and 47.29% for mass = 122.90 amu. Calculate the atomic mass of antimony. ...
... occurring isotopes with mass numbers of 121 and 123. The relative abundance and atomic masses are 57.12 % for mass = 120.90 amu, and 47.29% for mass = 122.90 amu. Calculate the atomic mass of antimony. ...
Ch 6.7 - Explaining the Atom
... - An isotope is an atom with the same number of protons (3) for lithium, but a different Atomic Mass, 7u for Lithium. - Mass Number of 7 u – 3 (Protons) = 4 neutrons in the nucleus. - The True Atomic Mass of Lithium is not a whole number but a combination of Lithium 6u and Lithium 7u, mass number of ...
... - An isotope is an atom with the same number of protons (3) for lithium, but a different Atomic Mass, 7u for Lithium. - Mass Number of 7 u – 3 (Protons) = 4 neutrons in the nucleus. - The True Atomic Mass of Lithium is not a whole number but a combination of Lithium 6u and Lithium 7u, mass number of ...
Radioactive Decay Mechanisms
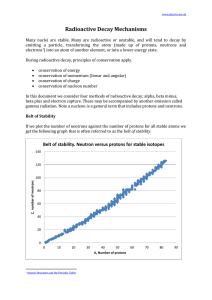
... 1. there are no stable nuclei with an atomic number of 84 or greater. 2. for smaller atoms the ratio neutrons:protons is 1:1 3. for larger atoms the ratio of neutrons:protons approaches about 1.5:1 Since the strong nuclear force weakens significantly with distance, as the atom gets bigger. If the nu ...
... 1. there are no stable nuclei with an atomic number of 84 or greater. 2. for smaller atoms the ratio neutrons:protons is 1:1 3. for larger atoms the ratio of neutrons:protons approaches about 1.5:1 Since the strong nuclear force weakens significantly with distance, as the atom gets bigger. If the nu ...
Atomic Theory: Skeleton Notes
... Performed famous “________ ________ Experiment” The experimental evidence that led to the Rutherford model was the results of bombarding a thin metal foil with an ________ particle (helium nuclei) beam. The beam was mostly____________, as expected; however, a small but significant number of alph ...
... Performed famous “________ ________ Experiment” The experimental evidence that led to the Rutherford model was the results of bombarding a thin metal foil with an ________ particle (helium nuclei) beam. The beam was mostly____________, as expected; however, a small but significant number of alph ...
UNIT 1 Atomic Structure
... Note electrons are usually shown as far apart as possible -they have the same charge and therefore repel each other. 3. The Noble gases (Group 0) have a stable electron configuration (s2p6) with 8 electrons filling the outer s and p orbitals. This stability comes from the low energy state of this c ...
... Note electrons are usually shown as far apart as possible -they have the same charge and therefore repel each other. 3. The Noble gases (Group 0) have a stable electron configuration (s2p6) with 8 electrons filling the outer s and p orbitals. This stability comes from the low energy state of this c ...
Document
... By definition, the mass of 12C is exactly 12 amu. Now all the present atoms are assigned according to C12 isotopes A 24Mg atom has a mass approximately twice that of the 12C atom, so its mass is 24 u. A 4He atom has a mass approximately 1/3 that of the 12C atom, so its mass is 4 u. 1H atom has a mas ...
... By definition, the mass of 12C is exactly 12 amu. Now all the present atoms are assigned according to C12 isotopes A 24Mg atom has a mass approximately twice that of the 12C atom, so its mass is 24 u. A 4He atom has a mass approximately 1/3 that of the 12C atom, so its mass is 4 u. 1H atom has a mas ...
ch-4-earth-chemistry
... Example: A neutral sodium atom has a charge of zero (equal # of protons and neutrons) and only 1 valence electron. Once it loses that valence electron, it will have 8 valence electrons and be stable and most likely, not gain or lose anymore electrons. What would be the charge on a sodium atom that l ...
... Example: A neutral sodium atom has a charge of zero (equal # of protons and neutrons) and only 1 valence electron. Once it loses that valence electron, it will have 8 valence electrons and be stable and most likely, not gain or lose anymore electrons. What would be the charge on a sodium atom that l ...
Atomic Structure - Madison Public Schools
... 2. To understand Rutherford’s experiment 3. To describe some important features of subatomic particles 4. To learn about the terms isotope, atomic number, and mass number 5. To understand the use of the symbol to describe a given atom ...
... 2. To understand Rutherford’s experiment 3. To describe some important features of subatomic particles 4. To learn about the terms isotope, atomic number, and mass number 5. To understand the use of the symbol to describe a given atom ...