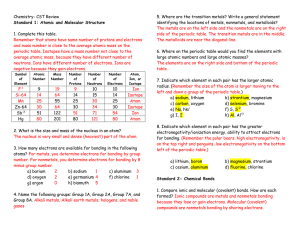
C4C5C6
... Ozone filters out and stops harmful ultraviolet light from reaching the surface of the earth CFCs were used as refrigerants and in aerosols because they have a low boiling point, are insoluble in water and are very unreactive. Use of CFCs in the UK is now banned to stop any more damage to the ozone ...
... Ozone filters out and stops harmful ultraviolet light from reaching the surface of the earth CFCs were used as refrigerants and in aerosols because they have a low boiling point, are insoluble in water and are very unreactive. Use of CFCs in the UK is now banned to stop any more damage to the ozone ...
Chemistry- CST Review
... 4. What atoms does carbon commonly form bonds with? Hydrogen, nitrogen, oxygen, and another carbon commonly form bonds with carbon. Standard 11- Nuclear Processes 1. What elements have radioactive isotopes? Elements with atomic number 84 and above are radioisotopes. There are more like carbon which ...
... 4. What atoms does carbon commonly form bonds with? Hydrogen, nitrogen, oxygen, and another carbon commonly form bonds with carbon. Standard 11- Nuclear Processes 1. What elements have radioactive isotopes? Elements with atomic number 84 and above are radioisotopes. There are more like carbon which ...
class slides for Chapter 42
... The independent particle model is based on the assumption that each nucleon remains in a well-defined quantum state within the nucleus and makes hardly any collisions at all! The nucleus, unlike the atom, has no fixed center of charge; we assume in this model that each nucleon moves in a potential w ...
... The independent particle model is based on the assumption that each nucleon remains in a well-defined quantum state within the nucleus and makes hardly any collisions at all! The nucleus, unlike the atom, has no fixed center of charge; we assume in this model that each nucleon moves in a potential w ...
Instructor`s Guide
... • The 2007 Nobel Prize in Physics was awarded to Albert Fert and Peter Grünberg for their work in spintronics (spintronics uses electron ‘spin’ to store information in technological devices). Their research made handheld music players such as the iPod possible. • ‘Killer electrons,’ found in the r ...
... • The 2007 Nobel Prize in Physics was awarded to Albert Fert and Peter Grünberg for their work in spintronics (spintronics uses electron ‘spin’ to store information in technological devices). Their research made handheld music players such as the iPod possible. • ‘Killer electrons,’ found in the r ...
Science 9 - Ms. J Reed
... 1. Electrons move around the nucleus in shells 2. Each shell is a certain distance away from the nucleus and can hold a definite number of electrons 3. After the shell closest to the nucleus is full, electrons start filling the next shell ...
... 1. Electrons move around the nucleus in shells 2. Each shell is a certain distance away from the nucleus and can hold a definite number of electrons 3. After the shell closest to the nucleus is full, electrons start filling the next shell ...
SCI 111
... • Two Rules – Write symbol for positive ion first followed by the symbol for the negative ion – Assign subscripts to assure compound is electrically neutral using cross-over technique ...
... • Two Rules – Write symbol for positive ion first followed by the symbol for the negative ion – Assign subscripts to assure compound is electrically neutral using cross-over technique ...
(a) Atoms - Warren County Schools
... more of the entire unit it appears in front of. The coefficient used in this example shows that, in the left reactant, there are 4 hydrogen, and in the product, there are 4 hydrogen and 2 oxygen. • The coefficient does not effect the oxygen in the reactant because it is not a compound with hydrogen ...
... more of the entire unit it appears in front of. The coefficient used in this example shows that, in the left reactant, there are 4 hydrogen, and in the product, there are 4 hydrogen and 2 oxygen. • The coefficient does not effect the oxygen in the reactant because it is not a compound with hydrogen ...
What is CHEMISTRY?
... It follows that if any value of r is allowed then so is any value of E. Movement of an electron from one level to another results in a change in its energy, ∆E. An energy change is related to frequency of electromagnetic radiation according to: ...
... It follows that if any value of r is allowed then so is any value of E. Movement of an electron from one level to another results in a change in its energy, ∆E. An energy change is related to frequency of electromagnetic radiation according to: ...
Chapter 2 Expanded Notes
... they retain this identity through chemical reactions. Atoms of one element are different from atoms of another element. However, all atoms of the same element have some identifying mark in common. Also note that of all the subatomic particles, only the proton did not change. All atoms have the same ...
... they retain this identity through chemical reactions. Atoms of one element are different from atoms of another element. However, all atoms of the same element have some identifying mark in common. Also note that of all the subatomic particles, only the proton did not change. All atoms have the same ...
Atoms Template
... particles, which were too small to be broken into smaller parts, atomos, meaning “uncuttable”. Democritus’ ideas were not widely believed because he had no evidence to support this hypothesis. ...
... particles, which were too small to be broken into smaller parts, atomos, meaning “uncuttable”. Democritus’ ideas were not widely believed because he had no evidence to support this hypothesis. ...
Investigating Atoms and Atomic Theory
... According to the modern atomic model, at atom has a small positively charged nucleus surrounded by a large region in which there are enough electrons to make an atom neutral. ...
... According to the modern atomic model, at atom has a small positively charged nucleus surrounded by a large region in which there are enough electrons to make an atom neutral. ...
Chapter 5
... Example: Naturally occurring Cu consists of 2 isotopes. It is 69.1% 63Cu with a mass of 62.9 amu, and 30.9% 65Cu, which has a mass of 64.9 amu. Calculate the atomic weight of Cu to one decimal place. ...
... Example: Naturally occurring Cu consists of 2 isotopes. It is 69.1% 63Cu with a mass of 62.9 amu, and 30.9% 65Cu, which has a mass of 64.9 amu. Calculate the atomic weight of Cu to one decimal place. ...
Campbell Biology, 10e (Reece) Chapter 2 The Chemical Context of
... A) the number of electrons in the element B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element 6) In what way are elements in the same column of the periodic table the same? They have the same number of _ ...
... A) the number of electrons in the element B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element 6) In what way are elements in the same column of the periodic table the same? They have the same number of _ ...
Select as many as apply.
... this family crosses the metal nonmetal barrier and thus would have different chemical properties 26Fe, 27Co, 28Ni 7N, 8O, 9F 16S, 34Se, 52Te These elements are all in the same chemical ...
... this family crosses the metal nonmetal barrier and thus would have different chemical properties 26Fe, 27Co, 28Ni 7N, 8O, 9F 16S, 34Se, 52Te These elements are all in the same chemical ...
CHAPTER 4
... Example: Naturally occurring Cu consists of 2 isotopes. It is 69.1% 63Cu with a mass of 62.9 amu, and 30.9% 65Cu, which has a mass of 64.9 amu. Calculate the atomic weight of Cu to one decimal place. ...
... Example: Naturally occurring Cu consists of 2 isotopes. It is 69.1% 63Cu with a mass of 62.9 amu, and 30.9% 65Cu, which has a mass of 64.9 amu. Calculate the atomic weight of Cu to one decimal place. ...
File
... nucleus of the atom with a definite fixed energy in a fixed path, without emitting or absorbing energy ◦ Based on his calculations and research with the atomic emission spectrum of Hydrogen ◦ His model of electron arrangement correctly predicted Hydrogen’s atomic spectrum ...
... nucleus of the atom with a definite fixed energy in a fixed path, without emitting or absorbing energy ◦ Based on his calculations and research with the atomic emission spectrum of Hydrogen ◦ His model of electron arrangement correctly predicted Hydrogen’s atomic spectrum ...
The five main types of redox reactions are combination
... Redox reactions are all around us. In fact, much of our technology, from fire to laptop batteries, is largely based on redox reactions. Redox (reductionoxidation) reactions are those in which the oxidation states of the reactants change. This occurs because in such reactions, electrons are always ...
... Redox reactions are all around us. In fact, much of our technology, from fire to laptop batteries, is largely based on redox reactions. Redox (reductionoxidation) reactions are those in which the oxidation states of the reactants change. This occurs because in such reactions, electrons are always ...
CHAPTER-4 STRUCTURE OF THE ATOM
... 2. If it is the outermost orbit, then it should have not more than 8 electrons. 3. There should be step-wise filling of electrons in different orbits, i.e., electrons are not accompanied in a given orbit if the earlier orbits or shells are incompletely filled. Q.7: Define valency by taking examples ...
... 2. If it is the outermost orbit, then it should have not more than 8 electrons. 3. There should be step-wise filling of electrons in different orbits, i.e., electrons are not accompanied in a given orbit if the earlier orbits or shells are incompletely filled. Q.7: Define valency by taking examples ...
History of the Atom Model
... indestructible, that atoms are in motion and that there are kinds of atoms, which differ in shape, and size. Dalton is the next scientist to make any significant change to the atom model. Actually, much of what Dalton did is to gather the ideas of his times about atoms and revise them as he saw fit. ...
... indestructible, that atoms are in motion and that there are kinds of atoms, which differ in shape, and size. Dalton is the next scientist to make any significant change to the atom model. Actually, much of what Dalton did is to gather the ideas of his times about atoms and revise them as he saw fit. ...
Atomic Structure
... • Electrons give off energy when they “jump” back down to the more stable ground state. • Ground State – when the electrons in an atom are arranged in the lowest possible energy level. ...
... • Electrons give off energy when they “jump” back down to the more stable ground state. • Ground State – when the electrons in an atom are arranged in the lowest possible energy level. ...
KENTUCKY TECH ELIZABETHTOWN
... Electrons are held tightly and are not given up easily Examples: Rubber Plastic Glass Wood ...
... Electrons are held tightly and are not given up easily Examples: Rubber Plastic Glass Wood ...
Chapter 2: Atoms, Molecules, and Ions
... 13. Avogadro's hypothesis states that: A) Each atom of oxygen is 16 times more massive than an atom of hydrogen. B) A given compound always contains exactly the same proportion of elements by mass. C) When two elements form a series of compounds, the ratios of masses that combine with 1 gram of the ...
... 13. Avogadro's hypothesis states that: A) Each atom of oxygen is 16 times more massive than an atom of hydrogen. B) A given compound always contains exactly the same proportion of elements by mass. C) When two elements form a series of compounds, the ratios of masses that combine with 1 gram of the ...
Unit 13 Worksheet Answers
... (a) HgO is added to the system increase (c) decrease the temperature of the system. increase (b) Hg is added to the system. decrease (d) the volume is decreased. No change 18) Predict the effect of decreasing the temperature on the position of the following equilibrium. (a) H 2 (g) + Cl 2 (g) ↔ 2HCl ...
... (a) HgO is added to the system increase (c) decrease the temperature of the system. increase (b) Hg is added to the system. decrease (d) the volume is decreased. No change 18) Predict the effect of decreasing the temperature on the position of the following equilibrium. (a) H 2 (g) + Cl 2 (g) ↔ 2HCl ...
Chemical Bonding I
... nuclei of the bonded atoms. • As with bond energies, these are averages since there are slight variaGons according to the molecular structure. • The next few slides give some typical values. • N ...
... nuclei of the bonded atoms. • As with bond energies, these are averages since there are slight variaGons according to the molecular structure. • The next few slides give some typical values. • N ...
Chemistry SOL Review Test
... a. the smallest particle of an element that retains the properties of that element b. atoms of the same element with different masses c. the number of protons in the nucleus of an atom d. attraction of positive nucleus for the outer negative electrons e. electrons in between the outer electrons and ...
... a. the smallest particle of an element that retains the properties of that element b. atoms of the same element with different masses c. the number of protons in the nucleus of an atom d. attraction of positive nucleus for the outer negative electrons e. electrons in between the outer electrons and ...