Atoms - Issaquah Connect
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
Chapter 4 Atomic Structure
... had a certain mass, size, and chemical behavior that was determined by what kind of element they were. ...
... had a certain mass, size, and chemical behavior that was determined by what kind of element they were. ...
Chapter 2 - Cloudfront.net
... temperature and pressure, compounds always react in whole number ratios by volume. Avogadro- interpreted that to mean: at the same temperature and pressure, equal volumes of gas contain the same number of particles (called Avogadro’s hypothesis) ...
... temperature and pressure, compounds always react in whole number ratios by volume. Avogadro- interpreted that to mean: at the same temperature and pressure, equal volumes of gas contain the same number of particles (called Avogadro’s hypothesis) ...
Kevin D - CSUN.edu
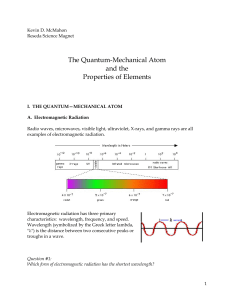
... the nucleus one obtains a nearly sphrical distribution of electrons around the nucleus with the greatest concentration approximately 0.529 Å away from the nucleus. There is no way of knowing where the electron is within the sphere because of uncertainty. Because of its wave nature the electron effec ...
... the nucleus one obtains a nearly sphrical distribution of electrons around the nucleus with the greatest concentration approximately 0.529 Å away from the nucleus. There is no way of knowing where the electron is within the sphere because of uncertainty. Because of its wave nature the electron effec ...
Unit 10
... Mg + O2 → ________________ Oxides of transition metals are usually coloured. No apparent reaction for silver, gold and platinum (unreactive metals). ...
... Mg + O2 → ________________ Oxides of transition metals are usually coloured. No apparent reaction for silver, gold and platinum (unreactive metals). ...
2011 Spring 1 key
... 8. The accepted SI base unit for energy is the joule with an abbreviation of J. 9. The chemical symbol for cobalt is Co, for potassium is K, and for silver is Ag. 10. The names of elements that correspond to the following symbols are sodium for Na, cadmium for Cd, and manganese for Mn. 11. The name ...
... 8. The accepted SI base unit for energy is the joule with an abbreviation of J. 9. The chemical symbol for cobalt is Co, for potassium is K, and for silver is Ag. 10. The names of elements that correspond to the following symbols are sodium for Na, cadmium for Cd, and manganese for Mn. 11. The name ...
Final Exam - Seattle Central College
... • Identify the type of intermolecular force for a molecule as London/dispersion forces, dipoledipole forces, hydrogen bonding, or ion-diple forces • Know that hydrogen bonds are the strongest type of intermolecular force, dipole-dipole forces are the next strongest, and London forces are generally t ...
... • Identify the type of intermolecular force for a molecule as London/dispersion forces, dipoledipole forces, hydrogen bonding, or ion-diple forces • Know that hydrogen bonds are the strongest type of intermolecular force, dipole-dipole forces are the next strongest, and London forces are generally t ...
Bonding practice lessons 1-3
... The results of these tests suggest that A) both solids contain only ionic bonds B) both solids contain only covalent bonds C) solid A contains only covalent bonds and solid B contains only ionic bonds D) solid A contains only ionic bonds and solid B contains only covalent bonds 22. The bonds between ...
... The results of these tests suggest that A) both solids contain only ionic bonds B) both solids contain only covalent bonds C) solid A contains only covalent bonds and solid B contains only ionic bonds D) solid A contains only ionic bonds and solid B contains only covalent bonds 22. The bonds between ...
2 Biochem I
... • Atoms (apples) are the individual items belonging to a specific element (variety) in the Periodic Table. • Atoms belonging to an element are very similar but not identical (they have slightly different masses) Three atoms (apples) from the variety (element) Golden Delicious ...
... • Atoms (apples) are the individual items belonging to a specific element (variety) in the Periodic Table. • Atoms belonging to an element are very similar but not identical (they have slightly different masses) Three atoms (apples) from the variety (element) Golden Delicious ...
Atomic Structure – Subatomic Particles
... The most famous detective in literature and history never existed. Sherlock Holmes was the creation of the British author Sir Arthur Conan Doyle. This mythical person had capabilities far beyond those of mere mortals. Holmes was capable of spotting the tiniest clue, the smallest piece of evidence to ...
... The most famous detective in literature and history never existed. Sherlock Holmes was the creation of the British author Sir Arthur Conan Doyle. This mythical person had capabilities far beyond those of mere mortals. Holmes was capable of spotting the tiniest clue, the smallest piece of evidence to ...
A is for atom
... Bohr’s Atom Bohr - brought the concept of quantization into atomic theory. Electrons could only move in certain specific orbits corresponding to specific amounts of energy. These ENERGY LEVELS radiated out from the nucleus with higher energies being further away. Electrons do not radiate energy in ...
... Bohr’s Atom Bohr - brought the concept of quantization into atomic theory. Electrons could only move in certain specific orbits corresponding to specific amounts of energy. These ENERGY LEVELS radiated out from the nucleus with higher energies being further away. Electrons do not radiate energy in ...
+ H 2 SO 4(aq) - Rothschild Science
... Single Replacement Reactions Reactivity of a metal makes a difference! If a metal is more reactive than the metal it is displacing a rxn will occur. If the metal is less reactive than the metal it is displacing, a rxn will not occur. ...
... Single Replacement Reactions Reactivity of a metal makes a difference! If a metal is more reactive than the metal it is displacing a rxn will occur. If the metal is less reactive than the metal it is displacing, a rxn will not occur. ...
Advanced Chemistry Midterm
... 35. A substance that cannot be separated into simpler substances by a chemical change is called a(n) a. compound b. mixture c. element d. crystal 36. Which of the following statements is part of Dalton’s atomic theory of matter? a. all atoms are identical b. all atoms of a given element are identica ...
... 35. A substance that cannot be separated into simpler substances by a chemical change is called a(n) a. compound b. mixture c. element d. crystal 36. Which of the following statements is part of Dalton’s atomic theory of matter? a. all atoms are identical b. all atoms of a given element are identica ...
The Atom
... Greeks settled disagreements by argument Aristotle was more famous He won His ideas carried through middle ages. Alchemists change lead to gold ...
... Greeks settled disagreements by argument Aristotle was more famous He won His ideas carried through middle ages. Alchemists change lead to gold ...
File - CToThe3Chemistry
... 4. All the isotopes of oxygen behave the same chemically. Which subatomic particles must be responsible for how an atom behaves chemically? Electrons are responsible for the behavior of atoms. 5. What part of Dalton’s theory does the discovery of isotopes prove wrong? Dalton said that atoms of the s ...
... 4. All the isotopes of oxygen behave the same chemically. Which subatomic particles must be responsible for how an atom behaves chemically? Electrons are responsible for the behavior of atoms. 5. What part of Dalton’s theory does the discovery of isotopes prove wrong? Dalton said that atoms of the s ...
Chapter 4 Atoms and Elements
... The number of electrons is given in the problem. The number of protons is obtained from the element’s atomic number in the periodic table (a) magnesium with atomic number 12 Ion charge = 12 – 10 = 2+ (Mg2+) (b) sulfur with atomic number 16 Ion charge = 16 – 18 = 2– (S2–) (c) iron with atomic number ...
... The number of electrons is given in the problem. The number of protons is obtained from the element’s atomic number in the periodic table (a) magnesium with atomic number 12 Ion charge = 12 – 10 = 2+ (Mg2+) (b) sulfur with atomic number 16 Ion charge = 16 – 18 = 2– (S2–) (c) iron with atomic number ...
Chemistry Chapter 3
... Dalton’s Atomic Theory All matter is made of atoms All atoms of any element are the same Atoms of different elements are different (size, mass, and other properties) Atoms of different elements can combine to form compounds In chemical reactions, atoms are not made, destroyed, or changed (subdivide ...
... Dalton’s Atomic Theory All matter is made of atoms All atoms of any element are the same Atoms of different elements are different (size, mass, and other properties) Atoms of different elements can combine to form compounds In chemical reactions, atoms are not made, destroyed, or changed (subdivide ...
Protons are the identity of an atom!
... Atoms of a particular element have a set number of protons. For example, every atom of hydrogen has one proton and every atom of gold has 79 protons. The number of protons is called the element’s atomic number. Atoms that are electrically neutral will have the same number of protons and electrons. I ...
... Atoms of a particular element have a set number of protons. For example, every atom of hydrogen has one proton and every atom of gold has 79 protons. The number of protons is called the element’s atomic number. Atoms that are electrically neutral will have the same number of protons and electrons. I ...
2014 Academic Challenge Sectional Chemistry Exam Solution Set 1
... C. At the same temperature, the sample with the highest vapor pressure will be the one with the weakest intermolecular forces. All of the molecules will have Van der Waals forces. B and D are also capable of hydrogen-bonding. A and E will have dipole-dipole interactions. Since C has only Van der Waa ...
... C. At the same temperature, the sample with the highest vapor pressure will be the one with the weakest intermolecular forces. All of the molecules will have Van der Waals forces. B and D are also capable of hydrogen-bonding. A and E will have dipole-dipole interactions. Since C has only Van der Waa ...
Chapter 2
... The atomic mass (A): total mass of protons + total mass of neutrons Atomic weight ~ Atomic mass # of protons are used to identify elements (Z) # of neutron are used to identify isotopes ( e.g. 14C6 and 12C6 ) Isotopes are written as follows: AXZ , i.e. 1H1, 2H1, 3H1 Chapter 2- ...
... The atomic mass (A): total mass of protons + total mass of neutrons Atomic weight ~ Atomic mass # of protons are used to identify elements (Z) # of neutron are used to identify isotopes ( e.g. 14C6 and 12C6 ) Isotopes are written as follows: AXZ , i.e. 1H1, 2H1, 3H1 Chapter 2- ...
Chemistry Unit Review
... b. Lead (II) iodide and potassium nitrate are produced when potassium iodide is added to lead (II) nitrate. ...
... b. Lead (II) iodide and potassium nitrate are produced when potassium iodide is added to lead (II) nitrate. ...
AP Chemistry Syllabus - Tuloso
... illuminate the principles. The following areas should be covered: A. Chemical reactivity and products of chemical reactions B. Relationships in the periodic table: horizontal, vertical, and diagonal with examples from alkali metals, alkaline earth metals, halogens, and the first series of transition ...
... illuminate the principles. The following areas should be covered: A. Chemical reactivity and products of chemical reactions B. Relationships in the periodic table: horizontal, vertical, and diagonal with examples from alkali metals, alkaline earth metals, halogens, and the first series of transition ...
2. Covalent network
... -Ionization Energy: energy required to remove an electron from an atom. The further the electron is from the nucleus, the easier it is to remove it. *Increases across a period *Decreases with increasing atomic # within a group -First ionization energy:energy it takes to remove the first electron fro ...
... -Ionization Energy: energy required to remove an electron from an atom. The further the electron is from the nucleus, the easier it is to remove it. *Increases across a period *Decreases with increasing atomic # within a group -First ionization energy:energy it takes to remove the first electron fro ...
C4C5C6
... Ozone filters out and stops harmful ultraviolet light from reaching the surface of the earth CFCs were used as refrigerants and in aerosols because they have a low boiling point, are insoluble in water and are very unreactive. Use of CFCs in the UK is now banned to stop any more damage to the ozone ...
... Ozone filters out and stops harmful ultraviolet light from reaching the surface of the earth CFCs were used as refrigerants and in aerosols because they have a low boiling point, are insoluble in water and are very unreactive. Use of CFCs in the UK is now banned to stop any more damage to the ozone ...