Lab Stuff - WW-P 4
... Identify each as an acid, base or neutral substance. If it is neutral indicate the kind of substance it is. Indicate the pH of the solution formed from this substance. ...
... Identify each as an acid, base or neutral substance. If it is neutral indicate the kind of substance it is. Indicate the pH of the solution formed from this substance. ...
chapter-7-engage-page-235-discovering-parts-of
... He combined data from his own scientific research with data from the research of other scientists to propose the atomic theory. Dalton’s atomic theory supported some of the ideas of Democritus. The Atom Today, scientists agree that matter is made of atoms with empty space between and within th ...
... He combined data from his own scientific research with data from the research of other scientists to propose the atomic theory. Dalton’s atomic theory supported some of the ideas of Democritus. The Atom Today, scientists agree that matter is made of atoms with empty space between and within th ...
Science Class 9 Notes – Atoms and Molecules
... (i) Cation : It is positively charged ion and is formed by the loss of electron from an atom e.g. H+, Na+, Ca2+, Al3+, NH4+ etc. (ii) Anion : It is negatively charged ion and is formed by the gain of electrons by an atom, e.g. Cl-, O2-, C-, F-, CO32- PO43- etc. 9. Valency : The combining power (or c ...
... (i) Cation : It is positively charged ion and is formed by the loss of electron from an atom e.g. H+, Na+, Ca2+, Al3+, NH4+ etc. (ii) Anion : It is negatively charged ion and is formed by the gain of electrons by an atom, e.g. Cl-, O2-, C-, F-, CO32- PO43- etc. 9. Valency : The combining power (or c ...
Spring 2009 Final Exam Review – Part 2
... 2. Find the % of each element in each substance in #1. 3. How many molecules are there in 24 grams of FeF3? 4. How many molecules are there in 450 grams of Na2SO4? 5. How many grams are there in 2.3 x 1024 atoms of silver? 6. How many grams are there in 7.4 x 1023 molecules of AgNO3? 7. ...
... 2. Find the % of each element in each substance in #1. 3. How many molecules are there in 24 grams of FeF3? 4. How many molecules are there in 450 grams of Na2SO4? 5. How many grams are there in 2.3 x 1024 atoms of silver? 6. How many grams are there in 7.4 x 1023 molecules of AgNO3? 7. ...
Topic 3 Note Outline
... numbers that are not integers. • For example, the atomic mass of Cl is often quoted on periodic tables as 35.5, and may be represented thus, 35.5Cl17. This does not mean that there are 17 protons, 17 electrons and 18.5 neutrons in an atom of chlorine. It is not possible to have a fraction of a neutr ...
... numbers that are not integers. • For example, the atomic mass of Cl is often quoted on periodic tables as 35.5, and may be represented thus, 35.5Cl17. This does not mean that there are 17 protons, 17 electrons and 18.5 neutrons in an atom of chlorine. It is not possible to have a fraction of a neutr ...
6.1 Models of the Atom
... actual weights of different atoms so atoms now were real enough that they could be weighed! Dalton joined the world of chemical experiments to the world of philosophical ideas. •For example, to find the weight of fluorine compared to hydrogen (the lightest atom), the experimental weight ratio is 1:1 ...
... actual weights of different atoms so atoms now were real enough that they could be weighed! Dalton joined the world of chemical experiments to the world of philosophical ideas. •For example, to find the weight of fluorine compared to hydrogen (the lightest atom), the experimental weight ratio is 1:1 ...
Summaries of Review Topics for AP Chemistry
... Rule #1: Identify and name acids: acids are covalent compounds which formulas start with H (except H2O and H2O2). Find their name in the “Names and Formulas of Acids” below. If the acid is made with a polyatomic ion, change the ending of the ion from –ate to –ic, or from –ite to –ous and add acid to ...
... Rule #1: Identify and name acids: acids are covalent compounds which formulas start with H (except H2O and H2O2). Find their name in the “Names and Formulas of Acids” below. If the acid is made with a polyatomic ion, change the ending of the ion from –ate to –ic, or from –ite to –ous and add acid to ...
Section 4.1
... A configuration is an arrangement of objects in a given space. Some configurations are more stable than others, meaning that they are less likely to change. ...
... A configuration is an arrangement of objects in a given space. Some configurations are more stable than others, meaning that they are less likely to change. ...
Final Exam Review- no solutions
... 33. Suppose a barometer is constructed using methanol as the liquid. Given that the density of Hg is 13.6 g/mL and that of methanol is 0.791 g/mL, and disregarding the effect of methanol gas molecules above methanol liquid, calculate the height of the methanol column at 1 atm of pressure and 0°C. Wh ...
... 33. Suppose a barometer is constructed using methanol as the liquid. Given that the density of Hg is 13.6 g/mL and that of methanol is 0.791 g/mL, and disregarding the effect of methanol gas molecules above methanol liquid, calculate the height of the methanol column at 1 atm of pressure and 0°C. Wh ...
Preview Sample 1
... 1. List several differences between ionic and covalent bonds. Ionic bonds occur when ions of opposite charge are mutually attracted. Acids and bases are examples of ionic compounds. Covalent bonds are strong chemical bonds that occur when atoms share electrons. Methane and sugar are examples of cova ...
... 1. List several differences between ionic and covalent bonds. Ionic bonds occur when ions of opposite charge are mutually attracted. Acids and bases are examples of ionic compounds. Covalent bonds are strong chemical bonds that occur when atoms share electrons. Methane and sugar are examples of cova ...
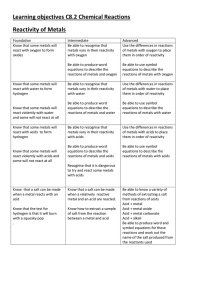
Learning objectives C8.2 Chemical Reactions Reactivity of Metals
... of a metal is linked to the method by which it is extracted Understand that the value of the voltage cell depends on the difference in reactivity of the 2 metals used ...
... of a metal is linked to the method by which it is extracted Understand that the value of the voltage cell depends on the difference in reactivity of the 2 metals used ...
View PDF
... 1. Atoms are tiny, invisible particles. 2. Atoms of one element are all the same. 3. Atoms of different elements are different. 4. Compounds form by combining atoms. ...
... 1. Atoms are tiny, invisible particles. 2. Atoms of one element are all the same. 3. Atoms of different elements are different. 4. Compounds form by combining atoms. ...
Final review packet
... 6. A given isotope has a half-life of 5.0 minutes. If the initial mass is 280 grams, how many grams will be left after 15 minutes? How many half-lives is this? 7. Write a balanced nuclear decay equation for each of the following: ...
... 6. A given isotope has a half-life of 5.0 minutes. If the initial mass is 280 grams, how many grams will be left after 15 minutes? How many half-lives is this? 7. Write a balanced nuclear decay equation for each of the following: ...
Models of the Atom - Central Magnet School
... • All the positive charge and almost all of the mass are concentrated in a small region in the center of the atom. • He called this tiny core the nucleus. ...
... • All the positive charge and almost all of the mass are concentrated in a small region in the center of the atom. • He called this tiny core the nucleus. ...
SAMPLE QUESTION PAPER-II Chemistry (Theory) Class-XII
... They prepare benzene diazonium chloride and stored it at room temperature. Due to holiday, they start preparing azodye but it cannot be prepared. Then their friend Reena told them to prepare benzene diazonium chloride again and to use it immediately to prepare azo dye and they proceed accordingly an ...
... They prepare benzene diazonium chloride and stored it at room temperature. Due to holiday, they start preparing azodye but it cannot be prepared. Then their friend Reena told them to prepare benzene diazonium chloride again and to use it immediately to prepare azo dye and they proceed accordingly an ...
Chemical Reactions
... Chemical reaction is the changing of substances to other substances by breaking bonds in reactants and forming new bonds in the products. -when some chemicals come into contact, they break apart, join, or rearrange to form new chemicals (always to become more stable) -produce new substances with new ...
... Chemical reaction is the changing of substances to other substances by breaking bonds in reactants and forming new bonds in the products. -when some chemicals come into contact, they break apart, join, or rearrange to form new chemicals (always to become more stable) -produce new substances with new ...
AP Chapter 6 Powerpoint
... • In many-electron atoms, the electron-electron repulsions cause the different subshells to ...
... • In many-electron atoms, the electron-electron repulsions cause the different subshells to ...
Unit 3 GROUP QUIZ
... b. Electrons are negatively charged and have a mass of 1 u. c. The nucleus of an atom is positively charged. d. The neutron is found in the nucleus of an atom. ___11. All atoms are ______. a. positively charged, with the number of protons exceeding the number of electrons. b. Negatively charged, wit ...
... b. Electrons are negatively charged and have a mass of 1 u. c. The nucleus of an atom is positively charged. d. The neutron is found in the nucleus of an atom. ___11. All atoms are ______. a. positively charged, with the number of protons exceeding the number of electrons. b. Negatively charged, wit ...
Chapter 3 Section 1 Notes
... What did Rutherford Propose? Rutherford proposed that most of the mass of the atom was in the atom’s center. An interactive model of Rutherford’s Gold-foil ...
... What did Rutherford Propose? Rutherford proposed that most of the mass of the atom was in the atom’s center. An interactive model of Rutherford’s Gold-foil ...
Thermodynamics Test Study Guide—AP _____ 1. The entropy
... reaction is equal to 1.00. ( Assume the ΔH and the ΔS are independent of temperature). c) Calculate the standard enthalpy change (ΔH) that occurs when 0.256 mol of NF3 (g) is formed from N2 (g) and F2 (g) at 1.00 atm and 298K. d) The enthalpy change in a chemical reaction is the difference betwe ...
... reaction is equal to 1.00. ( Assume the ΔH and the ΔS are independent of temperature). c) Calculate the standard enthalpy change (ΔH) that occurs when 0.256 mol of NF3 (g) is formed from N2 (g) and F2 (g) at 1.00 atm and 298K. d) The enthalpy change in a chemical reaction is the difference betwe ...
Up And Atom - Lesson Corner
... PATSY PARTIN AND ELANA JONES characteristics. Discuss the rationale for each answer. SAY, “Imagine taking a pure sample of an element, such as gold, and cutting it in half. Suppose you cut it in half again and again. You would finally have a piece so tiny that it could not be divided further and sti ...
... PATSY PARTIN AND ELANA JONES characteristics. Discuss the rationale for each answer. SAY, “Imagine taking a pure sample of an element, such as gold, and cutting it in half. Suppose you cut it in half again and again. You would finally have a piece so tiny that it could not be divided further and sti ...
2. Chapter 2
... Our Earth, the Sun, and everything else in our solar system, along with all the stars and galaxies beyond, contain an amazing variety of matter. You may recall that an element is a pure substance that cannot be broken down or separated into simpler substances. The reason an element cannot be broken ...
... Our Earth, the Sun, and everything else in our solar system, along with all the stars and galaxies beyond, contain an amazing variety of matter. You may recall that an element is a pure substance that cannot be broken down or separated into simpler substances. The reason an element cannot be broken ...