sample - Bright Red Publishing
... change involved when one mole of a substance is formed from its elements in their standard states. The standard state of a substance is its most stable form at a pressure of one atmosphere and at a specified temperature which is normally taken as 25°C or 298 K. It follows from the above definition t ...
... change involved when one mole of a substance is formed from its elements in their standard states. The standard state of a substance is its most stable form at a pressure of one atmosphere and at a specified temperature which is normally taken as 25°C or 298 K. It follows from the above definition t ...
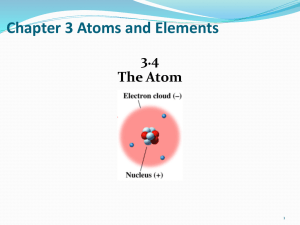
Chapter 3 Atoms and Elements
... • a proton has a mass of about 1 (1.007) amu. • a neutron has a mass of about 1 (1.008) amu. • an electron has a very small mass, 0.000 549 amu. ...
... • a proton has a mass of about 1 (1.007) amu. • a neutron has a mass of about 1 (1.008) amu. • an electron has a very small mass, 0.000 549 amu. ...
ppt notes
... different than A and BA element Atoms of element A and B can be can be physically chemically combined mixed together as a compound ...
... different than A and BA element Atoms of element A and B can be can be physically chemically combined mixed together as a compound ...
Topic 2 – Atomic Structure Notes
... These are all hydrogen atoms as they have 1 proton in their nucleus. The difference between the atoms is caused by different numbers of neutrons in the nuclei. The different number of neutrons affects the MASS NUMBERS of the atoms. These 3 different atoms of hydrogen are called ISOTOPES. ...
... These are all hydrogen atoms as they have 1 proton in their nucleus. The difference between the atoms is caused by different numbers of neutrons in the nuclei. The different number of neutrons affects the MASS NUMBERS of the atoms. These 3 different atoms of hydrogen are called ISOTOPES. ...
Unit 2 - The Atom 1-3.key
... neutrons of the atomic nucleus of each isotope varies. In the case of helium, both isotopes have two protons in their nuclei. However, helium-3 has one neutron, while helium-4 has two neutrons. Table 4 lists the four stable isotopes of lead. The least abundant of these isotopes is lead-204, while th ...
... neutrons of the atomic nucleus of each isotope varies. In the case of helium, both isotopes have two protons in their nuclei. However, helium-3 has one neutron, while helium-4 has two neutrons. Table 4 lists the four stable isotopes of lead. The least abundant of these isotopes is lead-204, while th ...
The History of Atomic Theory
... According to the theory of wave mechanics, electrons do not move about an atom in a definite path, like the planets around the sun. In fact, it is impossible to determine the exact location of an electron. The probable location of an electron is based on how much energy the electron has. Accor ...
... According to the theory of wave mechanics, electrons do not move about an atom in a definite path, like the planets around the sun. In fact, it is impossible to determine the exact location of an electron. The probable location of an electron is based on how much energy the electron has. Accor ...
atom
... All matter is made of atoms Atoms of same element have the same size, mass, and properties Atoms can’t be subdivided, created or destroyed Atoms of different elements combine in whole number ratios to make compounds In chemical reactions, atoms can be combined, separated, and rearranged. ...
... All matter is made of atoms Atoms of same element have the same size, mass, and properties Atoms can’t be subdivided, created or destroyed Atoms of different elements combine in whole number ratios to make compounds In chemical reactions, atoms can be combined, separated, and rearranged. ...
Lecture two
... • “bed check” for electrons • description on how are electrons organized around the nucleus of protons and neutrons • Bohr model: Nils Bohr proposed electrons “orbit” around the atom’s nucleus in specific energy levels or orbits (electron shells) – these shells have a specific energy level – closer ...
... • “bed check” for electrons • description on how are electrons organized around the nucleus of protons and neutrons • Bohr model: Nils Bohr proposed electrons “orbit” around the atom’s nucleus in specific energy levels or orbits (electron shells) – these shells have a specific energy level – closer ...
Document
... • “bed check” for electrons • description on how are electrons organized around the nucleus of protons and neutrons • Bohr model: Nils Bohr proposed electrons “orbit” around the atom’s nucleus in specific energy levels or orbits (electron shells) – these shells have a specific energy level – closer ...
... • “bed check” for electrons • description on how are electrons organized around the nucleus of protons and neutrons • Bohr model: Nils Bohr proposed electrons “orbit” around the atom’s nucleus in specific energy levels or orbits (electron shells) – these shells have a specific energy level – closer ...
Chapter 7
... 4. Catalysts – A catalyst is a substance that alters the rate of a chemical reaction without being permanently changed in the reaction. Catalysts change the activation energy of the reaction!!! Your body has thousands of catalysts called enzymes that God created to keep your body running smoothly! ...
... 4. Catalysts – A catalyst is a substance that alters the rate of a chemical reaction without being permanently changed in the reaction. Catalysts change the activation energy of the reaction!!! Your body has thousands of catalysts called enzymes that God created to keep your body running smoothly! ...
POGIL: Atomic Models and the Development of the Atom Model 1
... properties. * 3. Atoms of different elements differ in their physical and chemical properties. 4. Atoms of different elements combine in simple, whole number ratios to form compounds. 5. In chemical reactions, atoms are combined, separated, or rearranged, but never created, destroyed, or changed. *I ...
... properties. * 3. Atoms of different elements differ in their physical and chemical properties. 4. Atoms of different elements combine in simple, whole number ratios to form compounds. 5. In chemical reactions, atoms are combined, separated, or rearranged, but never created, destroyed, or changed. *I ...
Ernest Rutherford
... Ernest Rutherford Worked with Geiger and Marsden to prove that the nucleus is positive Hans Geiger Involved in a number of experiments that lead to Rutherford’s breakthrough theory of the atom Ernest Marsden Experiments to study the scattering of alpha particles by thin metal foils James Chadwick ...
... Ernest Rutherford Worked with Geiger and Marsden to prove that the nucleus is positive Hans Geiger Involved in a number of experiments that lead to Rutherford’s breakthrough theory of the atom Ernest Marsden Experiments to study the scattering of alpha particles by thin metal foils James Chadwick ...
Chemical Reactions are…
... Elements • Hydrogen; 2 atoms • Sulfur: 1 atom • Oxygen: 4 atoms 7 atoms total ...
... Elements • Hydrogen; 2 atoms • Sulfur: 1 atom • Oxygen: 4 atoms 7 atoms total ...
The Mole - My CCSD
... packets or quanta of specific size. – When a specific amount of energy was added to an atom, an electron could jump into a higher energy level. No more…no less! ...
... packets or quanta of specific size. – When a specific amount of energy was added to an atom, an electron could jump into a higher energy level. No more…no less! ...
ch8 - Otterville R-VI School District
... organize reactants and products Be sure to include symbols showing states of each reactant and product Be sure to write the correct formula ...
... organize reactants and products Be sure to include symbols showing states of each reactant and product Be sure to write the correct formula ...
Name: Per:______ The History of Atomic Theory Homework Due
... Many people helped develop the theory of atoms. The first was a Greek named Democritus in the 4th century BC. He proposed that all matter was made of tiny particles. Democritus thought that empty space filled the area between the solid atoms. That concept of the atom was unchanged for centuries. The ...
... Many people helped develop the theory of atoms. The first was a Greek named Democritus in the 4th century BC. He proposed that all matter was made of tiny particles. Democritus thought that empty space filled the area between the solid atoms. That concept of the atom was unchanged for centuries. The ...
Problem Class Questions for PHY008 Atomic and Nuclear Physics
... A19. Atoms are electrically neutral, so if they contain electrons they must also contain some positive charge, but where? Thomson assumed that this was uniformly distributed like a soup throughout the atom but this was only conjecture. To find out, in 1909 Rutherford fired positively charged helium ...
... A19. Atoms are electrically neutral, so if they contain electrons they must also contain some positive charge, but where? Thomson assumed that this was uniformly distributed like a soup throughout the atom but this was only conjecture. To find out, in 1909 Rutherford fired positively charged helium ...
UNIT 4 ATOMIC THEORY 1. Atomic theory: Dalton`s model
... Why do we use the relative atomic mass? The mass of an atom is too small to be measured in grams. We can only measure the mass of an atom by comparing its mass to the mass of another atom. ...
... Why do we use the relative atomic mass? The mass of an atom is too small to be measured in grams. We can only measure the mass of an atom by comparing its mass to the mass of another atom. ...
Types of Chemical Reactions
... only C, H, (and maybe O) is reacted with oxygen – usually called ...
... only C, H, (and maybe O) is reacted with oxygen – usually called ...
transcript for this video
... that we’re still - looking at the Periodic Table, looking at the transient relevance – to this day, trying to synthesise new and ever more complex elements. I really like this, but I thought the use of images could have been slightly better. We’ve got the current Periodic Table in here. What I thoug ...
... that we’re still - looking at the Periodic Table, looking at the transient relevance – to this day, trying to synthesise new and ever more complex elements. I really like this, but I thought the use of images could have been slightly better. We’ve got the current Periodic Table in here. What I thoug ...
Exam practice answers 5
... At the positive electrode the fuel (hydrogen gas) is converted to hydrogen ions: H2(g) 2H+(aq) + 2e– At the negative electrode, oxygen is converted to water by reaction with the hydrogen ions: ½O2(g) + 2H+(aq) + 2e– H2O(l) Overall, the equation is: H2(g) + ½O2(g) H2O(l) If an alkaline ca ...
... At the positive electrode the fuel (hydrogen gas) is converted to hydrogen ions: H2(g) 2H+(aq) + 2e– At the negative electrode, oxygen is converted to water by reaction with the hydrogen ions: ½O2(g) + 2H+(aq) + 2e– H2O(l) Overall, the equation is: H2(g) + ½O2(g) H2O(l) If an alkaline ca ...
Document
... 1. Work backward from the required reaction, using the reactants and products to know to manipulate the other given reactants at your disposal. 2. Reverse any reactions as needed to give the required reactants and products. ...
... 1. Work backward from the required reaction, using the reactants and products to know to manipulate the other given reactants at your disposal. 2. Reverse any reactions as needed to give the required reactants and products. ...
9.1 REDOX Introduction to Oxidation and Reduction
... These elements can have multiple oxidation states. So when we talk to someone about, say, ‘Iron Oxide., we also have to tell them is the iron’s oxidation number +2, +3 ...
... These elements can have multiple oxidation states. So when we talk to someone about, say, ‘Iron Oxide., we also have to tell them is the iron’s oxidation number +2, +3 ...