Atoms and The Periodic Table
... predicted the pattern of energies that can be produced by a hydrogen atom. In order to derive his equation, Bohr suggested that electrons could only exist in fixed energy or “quantized” energy orbits. Since electrons could only exist in these orbits, it was not possible for them to spiral into the n ...
... predicted the pattern of energies that can be produced by a hydrogen atom. In order to derive his equation, Bohr suggested that electrons could only exist in fixed energy or “quantized” energy orbits. Since electrons could only exist in these orbits, it was not possible for them to spiral into the n ...
Week 1 - School of Chemical Sciences
... web. A one-page summary of one paper is due in section (JACS communication format recommended). All papers will be discussed in section and a familiarity with each is expected and may be tested for on exams. Literature summaries should clearly and succinctly convey the principal objective, results, ...
... web. A one-page summary of one paper is due in section (JACS communication format recommended). All papers will be discussed in section and a familiarity with each is expected and may be tested for on exams. Literature summaries should clearly and succinctly convey the principal objective, results, ...
The p-Block Elements The p-Block Elements
... liquid states, it is associated through hydrogen bonds as in the case of water and that accounts for its higher melting and boiling points than expected on the basis of its molecular mass. The ammonia molecule is trigonal pyramidal with the nitrogen atom at the apex. It has three bond pairs and one ...
... liquid states, it is associated through hydrogen bonds as in the case of water and that accounts for its higher melting and boiling points than expected on the basis of its molecular mass. The ammonia molecule is trigonal pyramidal with the nitrogen atom at the apex. It has three bond pairs and one ...
Chemistry
... smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, the chemist John Dalton, revived the term when he suggested that each element was made up of unique atoms and the atoms of an element are all the same. At that time, there were about 35 known ...
... smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, the chemist John Dalton, revived the term when he suggested that each element was made up of unique atoms and the atoms of an element are all the same. At that time, there were about 35 known ...
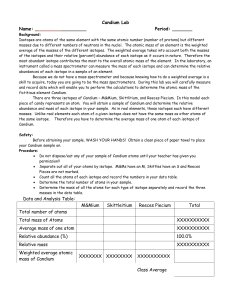
Candium Lab - OCPS TeacherPress
... 1.) Write a formula for each of the steps listed in calculations section. Example: Avg. mass of one atom = total mass of all atoms of an isotope total number of atoms of that isotope Relative Abundance = ...
... 1.) Write a formula for each of the steps listed in calculations section. Example: Avg. mass of one atom = total mass of all atoms of an isotope total number of atoms of that isotope Relative Abundance = ...
ouble Replacement or (Metathesis) Reactions
... forms at the negative electrode (cathode) and immediately undergoes reaction with water: ...
... forms at the negative electrode (cathode) and immediately undergoes reaction with water: ...
Problem Solving Drill - Rapid Learning Center
... Electrons have a charge of -1. Neutrons are neutral (no charge) and therefore do not affect the charge of the atom/ion. The correct answer is (C). ...
... Electrons have a charge of -1. Neutrons are neutral (no charge) and therefore do not affect the charge of the atom/ion. The correct answer is (C). ...
Revision IB2 Topic 1
... After heating, the stream of hydrogen gas was maintained until the apparatus had cooled. The following results were obtained. Mass of empty dish = 13.80 g Mass of dish and contents before heating = 21.75 g Mass of dish and contents after heating and leaving to cool = 20.15 g ...
... After heating, the stream of hydrogen gas was maintained until the apparatus had cooled. The following results were obtained. Mass of empty dish = 13.80 g Mass of dish and contents before heating = 21.75 g Mass of dish and contents after heating and leaving to cool = 20.15 g ...
MOLECULAR FORMULAS N C H H C N H HHH HH
... 4. (9 points) Gold, Au, is dissolved from rock by treating the rock with NaCN in the presence of oxygen. 4 Au(s) + 8 NaCN(aq) + O2(g) + 2 H2O(l) → 4 NaAu(CN)2(aq) + 4 NaOH(aq) (a) If you have 0.050 mol of gold, the number of moles of NaCN required is __________ mol and the number of moles of O2 requ ...
... 4. (9 points) Gold, Au, is dissolved from rock by treating the rock with NaCN in the presence of oxygen. 4 Au(s) + 8 NaCN(aq) + O2(g) + 2 H2O(l) → 4 NaAu(CN)2(aq) + 4 NaOH(aq) (a) If you have 0.050 mol of gold, the number of moles of NaCN required is __________ mol and the number of moles of O2 requ ...
2-1 The Nature of Matter
... The number of protons in an atom of an element is the element's atomic number. Carbon has 6 protons, so its atomic number is 6. More than 100 elements are known, but only about two dozen are commonly found in living organisms. ...
... The number of protons in an atom of an element is the element's atomic number. Carbon has 6 protons, so its atomic number is 6. More than 100 elements are known, but only about two dozen are commonly found in living organisms. ...
“History of the Atom: From Atomism to the Nuclear Model”
... the number of neutrons may increase differently from one atom to the next, and since elements may have different abundances of various isotopes (forms of the element with different numbers of neutrons), the average atomic mass may not always increase from one element to the next. This explains why M ...
... the number of neutrons may increase differently from one atom to the next, and since elements may have different abundances of various isotopes (forms of the element with different numbers of neutrons), the average atomic mass may not always increase from one element to the next. This explains why M ...
Chapter 17 Resource: Properties of Atoms and the Periodic Table
... Can you determine whether or not a solution is active? Can you put metals in order based on their activities? ...
... Can you determine whether or not a solution is active? Can you put metals in order based on their activities? ...
Section 2.5 The Modern View of Atomic Structure: An Introduction
... different elements are different in some fundamental way or ways. Chemical compounds are formed when atoms of different elements combine with each other. A given compound always has the same relative numbers and types of atoms. Chemical reactions involve reorganization of the atoms—changes in the wa ...
... different elements are different in some fundamental way or ways. Chemical compounds are formed when atoms of different elements combine with each other. A given compound always has the same relative numbers and types of atoms. Chemical reactions involve reorganization of the atoms—changes in the wa ...
Unit VIII Atoms… - VernonScienceLSA
... predicted the pattern of energies that can be produced by a hydrogen atom. In order to derive his equation, Bohr suggested that electrons could only exist in fixed energy or “quantized” energy orbits. Since electrons could only exist in these orbits, it was not possible for them to spiral into the n ...
... predicted the pattern of energies that can be produced by a hydrogen atom. In order to derive his equation, Bohr suggested that electrons could only exist in fixed energy or “quantized” energy orbits. Since electrons could only exist in these orbits, it was not possible for them to spiral into the n ...
CHEM 250Q
... Sodium (Na) reacts with sulfur (S) to form a compound in the ratio of two sodium atoms to one sulfur atom. Element X also reacts with sodium in the ratio of two sodium atoms to one element X atom. Which is most likely the identity of element X? A. ...
... Sodium (Na) reacts with sulfur (S) to form a compound in the ratio of two sodium atoms to one sulfur atom. Element X also reacts with sodium in the ratio of two sodium atoms to one element X atom. Which is most likely the identity of element X? A. ...
Chem 107 - Hughbanks Exam 1
... alphanumeric information. Print your name above, provide your UIN number, and sign the honor code statement below. ...
... alphanumeric information. Print your name above, provide your UIN number, and sign the honor code statement below. ...
File
... The oxidation number of an element indicates the number of electrons lost, gained or shared when it reacts and forms chemical bonds. The change in oxidation state of a species tells you if it has undergone oxidation or reduction. The usual Oxidation Numbers for common elements in their compounds ELE ...
... The oxidation number of an element indicates the number of electrons lost, gained or shared when it reacts and forms chemical bonds. The change in oxidation state of a species tells you if it has undergone oxidation or reduction. The usual Oxidation Numbers for common elements in their compounds ELE ...
O 95: Metal Substrates: Adsorption of Atoms and Inorganic Molecules
... Surfaces - Structure, Properties and Reactivity from Density Functional Theory — ∙Wolfgang Hieringer — Theoretical Chemistry, University of Erlangen-Nürnberg Transition metal coordination compounds adsorbed on metal surfaces have received increasing attention in recent years. Not only do they appear ...
... Surfaces - Structure, Properties and Reactivity from Density Functional Theory — ∙Wolfgang Hieringer — Theoretical Chemistry, University of Erlangen-Nürnberg Transition metal coordination compounds adsorbed on metal surfaces have received increasing attention in recent years. Not only do they appear ...
Document
... c) Recall: There are 2 situations of serious importance when dealing with oxidation states: i) We use these + and – oxidation states, when dealing with species of compounds or ions dissolved in water. In light of the definition of oxidation state, why do pure elements get an oxidation state of zer ...
... c) Recall: There are 2 situations of serious importance when dealing with oxidation states: i) We use these + and – oxidation states, when dealing with species of compounds or ions dissolved in water. In light of the definition of oxidation state, why do pure elements get an oxidation state of zer ...
CfE Higher Chemistry Unit 1 Chemical changes and structures
... the nucleus to attract the bonding electrons more strongly. Going down a group electronegativity decreases. As you move down a group in the periodic table, atoms increase in size, with a greater number of energy levels. The extra energy levels and increased covalent radius keep the bonding electrons ...
... the nucleus to attract the bonding electrons more strongly. Going down a group electronegativity decreases. As you move down a group in the periodic table, atoms increase in size, with a greater number of energy levels. The extra energy levels and increased covalent radius keep the bonding electrons ...
Chapter 6: Chemical Reactions – Study Guide
... 3. For each statement, write “yes” if evidence of a chemical reaction is present. Write “no” if there is no evidence of a chemical reaction. a) __________A tomato smells rotten. b) __________A drinking glass breaks into smaller pieces. c) __________A piece of ice melts. d) __________Drain cleaner is ...
... 3. For each statement, write “yes” if evidence of a chemical reaction is present. Write “no” if there is no evidence of a chemical reaction. a) __________A tomato smells rotten. b) __________A drinking glass breaks into smaller pieces. c) __________A piece of ice melts. d) __________Drain cleaner is ...
chem10chp7spr08
... Predict the product – has already been given, but we’ll learn how to do this later Write the correct chemical formulas – keep working on this __Al(s) + __Cl2(g) __AlCl3(s) Not mass balanced Balance equation using the correct stoich coefficients 1 Al & 2 Cl 1 Al & 3 Cl 2 Cl vs. 3 Cl: Find least c ...
... Predict the product – has already been given, but we’ll learn how to do this later Write the correct chemical formulas – keep working on this __Al(s) + __Cl2(g) __AlCl3(s) Not mass balanced Balance equation using the correct stoich coefficients 1 Al & 2 Cl 1 Al & 3 Cl 2 Cl vs. 3 Cl: Find least c ...
lectures on subjects in physics, chemistry and biology
... pressure it is found that the current is carried by electrons moving in one direction and positively charged atoms moving in the opposite direction. T h e stream of positively charged atoms can be allowed to pass through a hole in the negative electrode and so may be separated from the stream of ele ...
... pressure it is found that the current is carried by electrons moving in one direction and positively charged atoms moving in the opposite direction. T h e stream of positively charged atoms can be allowed to pass through a hole in the negative electrode and so may be separated from the stream of ele ...
ppt Lewis Dot Diagram Rules
... In general when there is a single central atom in the molecule, CH2ClF, SeCl2, O3 (CO2, NH3, PO43-), the central atom is the first atom in the chemical formula. Except when the first atom in the chemical formula is Hydrogen (H) or fluorine (F). In which case the central atom is the second atom in th ...
... In general when there is a single central atom in the molecule, CH2ClF, SeCl2, O3 (CO2, NH3, PO43-), the central atom is the first atom in the chemical formula. Except when the first atom in the chemical formula is Hydrogen (H) or fluorine (F). In which case the central atom is the second atom in th ...
Synthesis Reactions occur when two of more reactants combine to
... PROCEDURE: Perform reactions between each metal and each solution. Write down your observations. Use 3 drops of solution, just enough to cover the piece of metal. Do not perform a reaction of a metal with its own solution (ex. copper metal and CuCl2 solution)!! This creates unnecessary chemical wast ...
... PROCEDURE: Perform reactions between each metal and each solution. Write down your observations. Use 3 drops of solution, just enough to cover the piece of metal. Do not perform a reaction of a metal with its own solution (ex. copper metal and CuCl2 solution)!! This creates unnecessary chemical wast ...