Molar Mass - Science With Horne
... The mole (abbreviated mol) is the base unit for measuring the amount of a substance. The definition of a mole comes from how many particles (atoms, in this case) there is in exactly 12 grams of Carbon-12. Through many years of experimentation, it has been confirmed that a mole of any substance has 6 ...
... The mole (abbreviated mol) is the base unit for measuring the amount of a substance. The definition of a mole comes from how many particles (atoms, in this case) there is in exactly 12 grams of Carbon-12. Through many years of experimentation, it has been confirmed that a mole of any substance has 6 ...
Answers to Homework Problem Sheet 8
... Δ combH = -1421 kJ mol-1 Butane - the balanced equation for combustion is: ...
... Δ combH = -1421 kJ mol-1 Butane - the balanced equation for combustion is: ...
Chemistry Fall Final Study Guide Concepts
... 5. Using the periodic table, where are the metals and nonmetals? What is hydrogen? Metals are on the left side and the nonmetals are on the right side. Hydrogen, although on the left side of the periodic table, is an nonmetal. 6. Where are the alkali, alkaline earth, transition metals, halogens, and ...
... 5. Using the periodic table, where are the metals and nonmetals? What is hydrogen? Metals are on the left side and the nonmetals are on the right side. Hydrogen, although on the left side of the periodic table, is an nonmetal. 6. Where are the alkali, alkaline earth, transition metals, halogens, and ...
chapter 18 (moore) - Salisbury University
... Falling water (higher to lower potential energy) is a spontaneous process. As shown previously, H2 and O2 combine spontaneously to form water (exothermic) BUT … … liquid water vaporizes spontaneously at room temperature; an endothermic process. Conclusion: enthalpy alone is not a sufficient criteri ...
... Falling water (higher to lower potential energy) is a spontaneous process. As shown previously, H2 and O2 combine spontaneously to form water (exothermic) BUT … … liquid water vaporizes spontaneously at room temperature; an endothermic process. Conclusion: enthalpy alone is not a sufficient criteri ...
Ahmed Fazary_Click Chemistry
... Click chemistry is a concept introduced by K. Barry Sharpless in 2001 and describes chemistry tailored to generate substances quickly and reliably by joining small units together as nature does. In biochemistry, proteins are made from repeating amino acid units and sugars are made from repeating mon ...
... Click chemistry is a concept introduced by K. Barry Sharpless in 2001 and describes chemistry tailored to generate substances quickly and reliably by joining small units together as nature does. In biochemistry, proteins are made from repeating amino acid units and sugars are made from repeating mon ...
4_ Chemical reactions
... Oxidation Number: Electron Bookkeeping To determine whether electrons are transferred in a chemical reaction, we use a procedure that assigns an oxidation number to each atom in the reaction. Some Shortcut Methods for Assigning Oxidation Number to Atoms ...
... Oxidation Number: Electron Bookkeeping To determine whether electrons are transferred in a chemical reaction, we use a procedure that assigns an oxidation number to each atom in the reaction. Some Shortcut Methods for Assigning Oxidation Number to Atoms ...
Atomic Structure of Atoms
... Some Anomalies Some irregularities occur when there are enough electrons to half-fill s and d orbitals on a given row. ...
... Some Anomalies Some irregularities occur when there are enough electrons to half-fill s and d orbitals on a given row. ...
Chem G 9
... Students should appreciate that at the time that Dalton’s theory was proposed nothing was known about the internal structure of the atom. As a result of our increasing knowledge about atomic structure we now know that statement #2 and statement #3 are no longer true. Students should attempt to modif ...
... Students should appreciate that at the time that Dalton’s theory was proposed nothing was known about the internal structure of the atom. As a result of our increasing knowledge about atomic structure we now know that statement #2 and statement #3 are no longer true. Students should attempt to modif ...
File
... Elements are defined by the number of protons in an atom's nucleus. So, for example, an atom with 6 protons must be carbon and an atom with 92 protons must be uranium. If you change the protons, then you change the element. In addition to protons, the atoms of every element (except the simplest form ...
... Elements are defined by the number of protons in an atom's nucleus. So, for example, an atom with 6 protons must be carbon and an atom with 92 protons must be uranium. If you change the protons, then you change the element. In addition to protons, the atoms of every element (except the simplest form ...
5.7 Quantity Relationships in Chemical Reactions
... pieces of popcorn. In other words, not all the kernels “popped”. • What is the theoretical number of popcorn that we could expect? • What is the actual percent of the kernels popped? Note that in all the examples of chemical reactions given so far, it is assumed that each reaction works to perfectio ...
... pieces of popcorn. In other words, not all the kernels “popped”. • What is the theoretical number of popcorn that we could expect? • What is the actual percent of the kernels popped? Note that in all the examples of chemical reactions given so far, it is assumed that each reaction works to perfectio ...
The MOLE
... 1. What is the mass of 4.5 moles of Au? 2. How many atoms of Au will have a mass of 89.60g? 3. How many moles of gold do you have if you have 5.24 x 1026 atoms of gold? 4. What will be the mass of 5.24 x1026 atoms of Gold? ...
... 1. What is the mass of 4.5 moles of Au? 2. How many atoms of Au will have a mass of 89.60g? 3. How many moles of gold do you have if you have 5.24 x 1026 atoms of gold? 4. What will be the mass of 5.24 x1026 atoms of Gold? ...
Ch 01 notes
... Building on the Rutherford Atomic Model: The Nuclear Atom Model • The nuclear theory of the atom has three basic parts: 1. Most of the atom’s mass and all of its positive charge are contained in a small core called a nucleus. 2. Most of the volume of the atom is empty space, throughout which tiny, ...
... Building on the Rutherford Atomic Model: The Nuclear Atom Model • The nuclear theory of the atom has three basic parts: 1. Most of the atom’s mass and all of its positive charge are contained in a small core called a nucleus. 2. Most of the volume of the atom is empty space, throughout which tiny, ...
Chemistry - Birkenhead School
... Metals can be arranged in order of their reactivity in a reactivity series. The metals potassium, sodium, lithium, calcium, magnesium, zinc, iron and copper can be put in order of their reactivity from their reactions with water and dilute acids. The non-metals hydrogen and carbon are often included ...
... Metals can be arranged in order of their reactivity in a reactivity series. The metals potassium, sodium, lithium, calcium, magnesium, zinc, iron and copper can be put in order of their reactivity from their reactions with water and dilute acids. The non-metals hydrogen and carbon are often included ...
Chpt1
... Scientists use instruments (also called tools) to measure macroscopic properties of substances directly. For example, a ruler is used to measure the length of a sheet of paper. Microscopic properties cannot be measured as such; indirect methods have to be devised for these. Each measurement results ...
... Scientists use instruments (also called tools) to measure macroscopic properties of substances directly. For example, a ruler is used to measure the length of a sheet of paper. Microscopic properties cannot be measured as such; indirect methods have to be devised for these. Each measurement results ...
No Slide Title
... below. The series are listed in descending order of chemical reactivity, with the most active metals and halogens at the top (the elements most likely to undergo oxidation). Any metal on the list will replace the ions of those metals (to undergo reduction) that appear anywhere underneath it on the l ...
... below. The series are listed in descending order of chemical reactivity, with the most active metals and halogens at the top (the elements most likely to undergo oxidation). Any metal on the list will replace the ions of those metals (to undergo reduction) that appear anywhere underneath it on the l ...
Atoms and Molecules
... Molecules are made of atoms, so the relative mass of molecule can be calculated by adding together the atomic weights of the atoms that make up the molecule. MOLECULAR WEIGHT is the relative mass of a molecule expressed in atomic mass units (µ) and calculated by adding together the atomic weights of ...
... Molecules are made of atoms, so the relative mass of molecule can be calculated by adding together the atomic weights of the atoms that make up the molecule. MOLECULAR WEIGHT is the relative mass of a molecule expressed in atomic mass units (µ) and calculated by adding together the atomic weights of ...
Development of the Atomic Theory
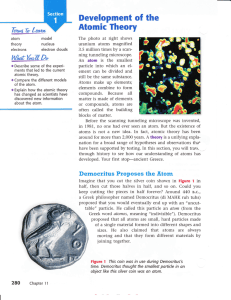
... the nucleus, as shown in the model in Figure tI. This is an atom of the element helium. Building Bigger Atoms You could build a carbon atom using 6 protons, 6 neutrons, and 6 electrons; or you could build an oxygen atom using 8 protons, 9 neutrons, and 8 electrons. You could even build a gold atom w ...
... the nucleus, as shown in the model in Figure tI. This is an atom of the element helium. Building Bigger Atoms You could build a carbon atom using 6 protons, 6 neutrons, and 6 electrons; or you could build an oxygen atom using 8 protons, 9 neutrons, and 8 electrons. You could even build a gold atom w ...
AP® Chemistry 2009 Free-Response Questions - AP Central
... (i) Write the electron configuration (e.g., 1s 2 2s 2 . . .) of each species. (ii) Explain why the radius of the S2− ion is larger than the radius of the S atom. (iii) Which of the two species would be attracted into a magnetic field? Explain. (b) The S2− ion is isoelectronic with the Ar atom. From ...
... (i) Write the electron configuration (e.g., 1s 2 2s 2 . . .) of each species. (ii) Explain why the radius of the S2− ion is larger than the radius of the S atom. (iii) Which of the two species would be attracted into a magnetic field? Explain. (b) The S2− ion is isoelectronic with the Ar atom. From ...
What are atoms?
... • Atoms are made of protons, neutrons, and electrons. • Protons are positively charged particles. • The mass of a proton is given in the atomic mass unit (u). One proton has a mass of 1 u. • Help make up the nucleus of the atom • Help identify the atom (could be considered an atom’s DNA) ...
... • Atoms are made of protons, neutrons, and electrons. • Protons are positively charged particles. • The mass of a proton is given in the atomic mass unit (u). One proton has a mass of 1 u. • Help make up the nucleus of the atom • Help identify the atom (could be considered an atom’s DNA) ...
CH 4 Notes
... When a substance loses electrons, it undergoes oxidation: Ca (s) + 2 H1+ (aq) ---> Ca2+ (aq) + H2 (g) The neutral Ca has lost two electrons to 2 H1+ to become Ca2+ We say Ca has been oxidized to Ca2+ When a substance gains electrons, it undergoes reduction: 2 Ca (s) + O2 (g) ---> 2 CaO (s) ...
... When a substance loses electrons, it undergoes oxidation: Ca (s) + 2 H1+ (aq) ---> Ca2+ (aq) + H2 (g) The neutral Ca has lost two electrons to 2 H1+ to become Ca2+ We say Ca has been oxidized to Ca2+ When a substance gains electrons, it undergoes reduction: 2 Ca (s) + O2 (g) ---> 2 CaO (s) ...
Scheme of work
... usually the most harmful when outside the body and alpha are the most dangerous when inside the body in terms of penetration of the radiation. ...
... usually the most harmful when outside the body and alpha are the most dangerous when inside the body in terms of penetration of the radiation. ...
C5-Early-Atomic-Theory-and-Structure-Comp
... 1. Elements are composed of minute, indivisible particles called atoms. – Atoms are made up of smaller particles 2. Atoms of the same element are alike in mass and size. – Isotopes of elements exist 3. Atoms of different elements have different masses and sizes. – Isotopes like C-14 and N-14 make th ...
... 1. Elements are composed of minute, indivisible particles called atoms. – Atoms are made up of smaller particles 2. Atoms of the same element are alike in mass and size. – Isotopes of elements exist 3. Atoms of different elements have different masses and sizes. – Isotopes like C-14 and N-14 make th ...
chapt 1 - Cantt Academy, Tahli Mohri Chowk, Rawalpindi
... convert cheap metals in to gold. They performed many experiment but could not succeed and wasted their time and money. These scientists are called alchemists and this branch of chemistry is called alchemy. However during that period these scientist discovered many new processes such as distillation, ...
... convert cheap metals in to gold. They performed many experiment but could not succeed and wasted their time and money. These scientists are called alchemists and this branch of chemistry is called alchemy. However during that period these scientist discovered many new processes such as distillation, ...