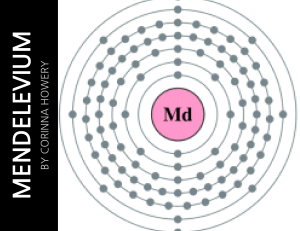
Mendelevium
... table so its atomic number is 101. There are 101 protons/electrons in the nucleus and 157 neutrons. It also has 2 valence electrons. Mendelevium has 7 shells. On the periodic table, mendelevium is in the group actinide and it is radioactive. Mendeleviums state of matter is radioactive. ...
... table so its atomic number is 101. There are 101 protons/electrons in the nucleus and 157 neutrons. It also has 2 valence electrons. Mendelevium has 7 shells. On the periodic table, mendelevium is in the group actinide and it is radioactive. Mendeleviums state of matter is radioactive. ...
Atom Basics
... 16. What subatomic (smaller than an atom) particles are protons and neutrons made from? ...
... 16. What subatomic (smaller than an atom) particles are protons and neutrons made from? ...
Atoms and Atomic Theory
... states that it is impossible to know both the location and speed of an electron at the same time, scientists developed what is known as the charge-cloud model. According to this model, electrons move around the nucleus, but no attempt is made to show the orbital paths of the electrons. The electrons ...
... states that it is impossible to know both the location and speed of an electron at the same time, scientists developed what is known as the charge-cloud model. According to this model, electrons move around the nucleus, but no attempt is made to show the orbital paths of the electrons. The electrons ...
Atomic Theories and Models - MrD-Home
... • The number of __________ found in the nucleus of an atom • Atoms have no overall electric charge (i.e. charge of atom is zero) ...
... • The number of __________ found in the nucleus of an atom • Atoms have no overall electric charge (i.e. charge of atom is zero) ...
Mileposts on the road to the atom
... Without knowledge of atomic mass, impossible to know how many atoms of one element combine with another Essential to know number of atoms to understand chemistry – write chemical formulae Atomic weight scale, largely enabled by Avogadro, provides link between experimental observables and numbers of ...
... Without knowledge of atomic mass, impossible to know how many atoms of one element combine with another Essential to know number of atoms to understand chemistry – write chemical formulae Atomic weight scale, largely enabled by Avogadro, provides link between experimental observables and numbers of ...
In an atom
... first electron shell would be about one kilometer from the golf ball, the second shell about four kilometers, the third nine kilometers and so on. If you find that hard to visualize then try this. The period at the end of this sentence, (depending on your monitor and the font you are using), is prob ...
... first electron shell would be about one kilometer from the golf ball, the second shell about four kilometers, the third nine kilometers and so on. If you find that hard to visualize then try this. The period at the end of this sentence, (depending on your monitor and the font you are using), is prob ...
4.1 Studying the Atom Notes
... 400 BC Greeks proposed some ideas of matter. o All things are made of “atoms” *** o Atoms are indivisible o Atoms are very small *** The word “atom” comes from Greek.: ...
... 400 BC Greeks proposed some ideas of matter. o All things are made of “atoms” *** o Atoms are indivisible o Atoms are very small *** The word “atom” comes from Greek.: ...
4 Atomic Structure / Quantum Mechanics
... – Energy produced form the shorter λ pushes the e- away from the nucleus ...
... – Energy produced form the shorter λ pushes the e- away from the nucleus ...
Atoms
... The number of protons does not change in an ion The number of neutrons does not change in an ions So, both the atomic number and the atomic mass remain the same. ...
... The number of protons does not change in an ion The number of neutrons does not change in an ions So, both the atomic number and the atomic mass remain the same. ...
ATOM WEBQUEST
... Atom Basics: Go to: http://www.chemtutor.com/struct.html and read the “And you thought you were strange” section to answer the following questions (put answers in the table). 1. What are the three subatomic particles that all atoms are made of? 2. Where are each of the three particles located within ...
... Atom Basics: Go to: http://www.chemtutor.com/struct.html and read the “And you thought you were strange” section to answer the following questions (put answers in the table). 1. What are the three subatomic particles that all atoms are made of? 2. Where are each of the three particles located within ...
syllabus details - hrsbstaff.ednet.ns.ca
... Explanations are only required for the first 20 elements, although general principles can extend to the whole of the periodic table. For example, students should know or be able to predict that K is in group I is using Z = 19, but need only know that since Cs is in group I, it has one electron in it ...
... Explanations are only required for the first 20 elements, although general principles can extend to the whole of the periodic table. For example, students should know or be able to predict that K is in group I is using Z = 19, but need only know that since Cs is in group I, it has one electron in it ...
chemical foundations, 020916
... living cell or organism that are necessary for the maintenance of life. In metabolism, some substances are broken down to yield energy for vital processes while other substances, necessary for life, are synthesized. ...
... living cell or organism that are necessary for the maintenance of life. In metabolism, some substances are broken down to yield energy for vital processes while other substances, necessary for life, are synthesized. ...
the atomic theory - Hackettstown School District
... •Lining up 100,000,000 copper atoms side by side would produce a line 1 cm long ...
... •Lining up 100,000,000 copper atoms side by side would produce a line 1 cm long ...
Electron Configurations
... Exceptions to the Aufbau Principle • Remember d and f orbitals require LARGE amounts of energy • If we can’t fill these sublevels, then the next best thing is to be HALF full (one electron in each orbital in the sublevel) • There are many exceptions, but the most common ones are d4 and d9 For the pu ...
... Exceptions to the Aufbau Principle • Remember d and f orbitals require LARGE amounts of energy • If we can’t fill these sublevels, then the next best thing is to be HALF full (one electron in each orbital in the sublevel) • There are many exceptions, but the most common ones are d4 and d9 For the pu ...
File
... Over the past few days you have learned about the subatomic particles in atoms and their charges. We have also viewed a few ideas in video form of how to go about building a model of an atom. In class, I will provide large beads, wire, string, hot glue gun, paint, and other basic supplies to build y ...
... Over the past few days you have learned about the subatomic particles in atoms and their charges. We have also viewed a few ideas in video form of how to go about building a model of an atom. In class, I will provide large beads, wire, string, hot glue gun, paint, and other basic supplies to build y ...
Chemical Reactions
... Rules for Writing and Balancing Equations 1. Determine the correct formulas for all 4. Balance the elements one at a time by the reactants and products. using coefficients. When no coefficient is written, it is assumed to be 1. Begin by 2. Write the skeleton equation by placing the formulas for the ...
... Rules for Writing and Balancing Equations 1. Determine the correct formulas for all 4. Balance the elements one at a time by the reactants and products. using coefficients. When no coefficient is written, it is assumed to be 1. Begin by 2. Write the skeleton equation by placing the formulas for the ...
File - CToThe3Chemistry
... 4. All the isotopes of oxygen behave the same chemically. Which subatomic particles must be responsible for how an atom behaves chemically? Electrons are responsible for the behavior of atoms. 5. What part of Dalton’s theory does the discovery of isotopes prove wrong? Dalton said that atoms of the s ...
... 4. All the isotopes of oxygen behave the same chemically. Which subatomic particles must be responsible for how an atom behaves chemically? Electrons are responsible for the behavior of atoms. 5. What part of Dalton’s theory does the discovery of isotopes prove wrong? Dalton said that atoms of the s ...
Presentation
... The charges on transition metal ions must be indicated. One method is shown here: The Roman numeral (stock system) gives the charge (oxidation number) of the cation: Ex: Nickel (II) is Ni+2 Nickel (III) is Ni+3 ...
... The charges on transition metal ions must be indicated. One method is shown here: The Roman numeral (stock system) gives the charge (oxidation number) of the cation: Ex: Nickel (II) is Ni+2 Nickel (III) is Ni+3 ...
Student Copy Study Guide Introduction to Periodic
... Identify the choice that best completes the statement or answers the question. 12. The modern periodic table is arranged in order of increasing atomic ____. a. mass b. charge c. number d. radius 13. Which of the following categories includes the majority of the elements? a. metalloids b. liquids c. ...
... Identify the choice that best completes the statement or answers the question. 12. The modern periodic table is arranged in order of increasing atomic ____. a. mass b. charge c. number d. radius 13. Which of the following categories includes the majority of the elements? a. metalloids b. liquids c. ...
AP Syllabus
... time is also available once a week. We do this during zero periods for those that do not have the optional zero period course, or after school for those that cannot make the zero hour. Scheduling of AP chemistry is also planned so that we can work through lunch if we need to in order to complete lab ...
... time is also available once a week. We do this during zero periods for those that do not have the optional zero period course, or after school for those that cannot make the zero hour. Scheduling of AP chemistry is also planned so that we can work through lunch if we need to in order to complete lab ...
$doc.title
... with one oxygen atom to form one molecule of water. On the atomic scale, we never see an example of one and a half hydrogen atoms combining with an oxygen atom. This was one of the first observations of the early chemists who explored the properties of chemical elements. This observation is known as ...
... with one oxygen atom to form one molecule of water. On the atomic scale, we never see an example of one and a half hydrogen atoms combining with an oxygen atom. This was one of the first observations of the early chemists who explored the properties of chemical elements. This observation is known as ...
Chem312 Au03 Problem Set 4
... when the electrons are in different orbitals with their spins pointing in the same direction (e.g., all spin up, ↑↑). It is higher energy if the electrons pair in one orbital or even if they have opposite sign (↑↓) in two different orbitals (because when the electrons have opposite spin they can get ...
... when the electrons are in different orbitals with their spins pointing in the same direction (e.g., all spin up, ↑↑). It is higher energy if the electrons pair in one orbital or even if they have opposite sign (↑↓) in two different orbitals (because when the electrons have opposite spin they can get ...























