p,d,f
... small that they are difficult to study directly; atomic models are constructed to explain experimental data on collections of atoms. ...
... small that they are difficult to study directly; atomic models are constructed to explain experimental data on collections of atoms. ...
Stoichiometry Review Package Answer Key
... For a visual practice on balancing equations and limiting/excess reagents go to: https://phet.colorado.edu/en/simulation/balancing-chemical-equations https://phet.colorado.edu/en/simulation/reactants-products-and-leftovers The following questions are based on the material covered so far. I will post ...
... For a visual practice on balancing equations and limiting/excess reagents go to: https://phet.colorado.edu/en/simulation/balancing-chemical-equations https://phet.colorado.edu/en/simulation/reactants-products-and-leftovers The following questions are based on the material covered so far. I will post ...
- Dr.Divan Fard
... Groups on the Periodic Table • On the periodic table, each vertical column is called a group of elements. • A group contains elements with similar chemical and physical properties. • Each group is identified by a group number at the top of the column. • The representative elements have group number ...
... Groups on the Periodic Table • On the periodic table, each vertical column is called a group of elements. • A group contains elements with similar chemical and physical properties. • Each group is identified by a group number at the top of the column. • The representative elements have group number ...
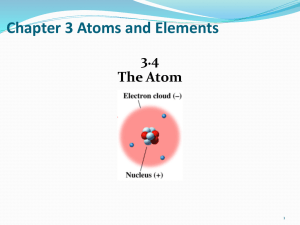
Chapter 3 Atoms and Elements
... • of an element is electrically neutral; the net charge of an atom is zero. • has an equal number of protons and electrons. number of protons = number of electrons Aluminum has 13 protons and 13 electrons. The net (overall) charge is zero. 13 protons (13+) + 13 electrons (13 -) = 0 ...
... • of an element is electrically neutral; the net charge of an atom is zero. • has an equal number of protons and electrons. number of protons = number of electrons Aluminum has 13 protons and 13 electrons. The net (overall) charge is zero. 13 protons (13+) + 13 electrons (13 -) = 0 ...
Dalton`s Personal Life
... • Einstein developed the mass-energy equivalence equation equation in 1905 • E = energy, m = mass, c = speed of light • Shows that when atom is converted into energy, it still produces a large amount of energy despite it’s small mass • Based on the facts that: – Laws of physics are the same in all i ...
... • Einstein developed the mass-energy equivalence equation equation in 1905 • E = energy, m = mass, c = speed of light • Shows that when atom is converted into energy, it still produces a large amount of energy despite it’s small mass • Based on the facts that: – Laws of physics are the same in all i ...
Name - Piscataway High School
... In the first week, she studied daily for 15 minutes and her end of the week test scores were 60%. During the second week, she studied daily for 30 minutes and her end of the week test scores were 70%. During the third week, she studied for 45 minutes and her end of the week test scores were 80%. Fin ...
... In the first week, she studied daily for 15 minutes and her end of the week test scores were 60%. During the second week, she studied daily for 30 minutes and her end of the week test scores were 70%. During the third week, she studied for 45 minutes and her end of the week test scores were 80%. Fin ...
PowerPoint Lectures - Northwest ISD Moodle
... • Different metals vary in how easily they are oxidized (how easily they lose electrons) • Activity series – list of metals arranged in order of decreasing ease of oxidation - Allows us to predict whether a metal will be oxidized or not • Any neutral metal (or hydrogen) on the list can be oxidized b ...
... • Different metals vary in how easily they are oxidized (how easily they lose electrons) • Activity series – list of metals arranged in order of decreasing ease of oxidation - Allows us to predict whether a metal will be oxidized or not • Any neutral metal (or hydrogen) on the list can be oxidized b ...
electrons - Bryant School District
... • An electron’s exact location cannot be determined. • It is impossible to determine both the exact location of an electron in an atom and the electron’s speed and direction. • The best scientists can do is calculate the chance of finding an electron in a certain place within an atom. ...
... • An electron’s exact location cannot be determined. • It is impossible to determine both the exact location of an electron in an atom and the electron’s speed and direction. • The best scientists can do is calculate the chance of finding an electron in a certain place within an atom. ...
Chapter 5 Review - Net Start Class
... Thomson discovered that electrons have a very_____ mass. small ...
... Thomson discovered that electrons have a very_____ mass. small ...
Chapter 11 section 2 questions - the atom
... gained electrons during the bonding process). More on this as we progress:) ...
... gained electrons during the bonding process). More on this as we progress:) ...
Atomic Model Stations - Moore Public Schools
... 2. What are the three basic parts of an atom? _____________________________________ ...
... 2. What are the three basic parts of an atom? _____________________________________ ...
Chem. 121, Sec 11 Name: Student I.D. Please Show Your Work
... 17. Choose the paramagnetic atom or ion: Ca, Ne, Sc3+, Cl-, Na. Show Orbital diagrams. (5 marks) ...
... 17. Choose the paramagnetic atom or ion: Ca, Ne, Sc3+, Cl-, Na. Show Orbital diagrams. (5 marks) ...
Section 1-2 Matter and Its Properties
... A chemical property relates to a substance’s ability to undergo changes that transform it into different substances. An example would be the ability of charcoal to burn in air to form carbon dioxide, and iron rusting to form iron oxide. A chemical change is a change in which one or more substances ...
... A chemical property relates to a substance’s ability to undergo changes that transform it into different substances. An example would be the ability of charcoal to burn in air to form carbon dioxide, and iron rusting to form iron oxide. A chemical change is a change in which one or more substances ...
Practice Test 1 (Chapters 1-7)
... 26. Which of the following processes is a chemical e. 6 change? 32. Which atomic particle determines the chemical a. Dry ice sublimes when left on the demo behavior of an atom? table in lecture. a. proton b. The light on a candle burns until a bell jar is b. electron placed over it for a period of t ...
... 26. Which of the following processes is a chemical e. 6 change? 32. Which atomic particle determines the chemical a. Dry ice sublimes when left on the demo behavior of an atom? table in lecture. a. proton b. The light on a candle burns until a bell jar is b. electron placed over it for a period of t ...
Chapter 3 Atoms and Elements
... • Use atomic mass and percent of each isotope to calculate the contribution of each isotope to the weighted average. Atomic mass 35Cl x % abundance = Atomic mass 37Cl x % abundance = • Sum is atomic mass of Cl is ...
... • Use atomic mass and percent of each isotope to calculate the contribution of each isotope to the weighted average. Atomic mass 35Cl x % abundance = Atomic mass 37Cl x % abundance = • Sum is atomic mass of Cl is ...
Chemistry Entrance Material for Grade 11 to 12 Answer Key
... [-D-] it boils when heated in air Effect of temperature on vapour pressure 05. How does the vapour pressure of a liquid vary with temperature( T)? As T increase the vapor pressure increase. When a liquid boils it absorbs heat at constant temperature 06. When a liquid at its boiling point is heated, ...
... [-D-] it boils when heated in air Effect of temperature on vapour pressure 05. How does the vapour pressure of a liquid vary with temperature( T)? As T increase the vapor pressure increase. When a liquid boils it absorbs heat at constant temperature 06. When a liquid at its boiling point is heated, ...
Answers to End-of-Chapter Questions – Brooker et al ARIS site
... 1. Before the experiment conducted by Ernest Rutherford, how did many scientists envision the structure of an atom? Answer: Scientists were aware that atoms contained charged particles. Many believed that the positive charges and mass were evenly distributed throughout the atom. 2. What was the hypo ...
... 1. Before the experiment conducted by Ernest Rutherford, how did many scientists envision the structure of an atom? Answer: Scientists were aware that atoms contained charged particles. Many believed that the positive charges and mass were evenly distributed throughout the atom. 2. What was the hypo ...
chemical reaction
... Chemical reactions are described by chemical equations. A chemical equation represents, with symbols and formulas, the identities and relative molecular or molar amounts of the reactants and products in a chemical reaction. For example, the following chemical equation shows that the reactant ammoni ...
... Chemical reactions are described by chemical equations. A chemical equation represents, with symbols and formulas, the identities and relative molecular or molar amounts of the reactants and products in a chemical reaction. For example, the following chemical equation shows that the reactant ammoni ...
Chapter 2 Introduction to Chemistry
... A substance is a particular kind of matter that has a uniform and definite composition. Pure substances contain only one kind of matter ...
... A substance is a particular kind of matter that has a uniform and definite composition. Pure substances contain only one kind of matter ...
Webquest - TeacherWeb
... 2. In what date was it determined that matter can neither be created nor destroyed. Name the date and the scientist. 3. Name the date and inventor of the modern version of the Atomic Theory. 4. I was born in 1831 and showed that electricity and magnetism are scientifically related. 5. He developed t ...
... 2. In what date was it determined that matter can neither be created nor destroyed. Name the date and the scientist. 3. Name the date and inventor of the modern version of the Atomic Theory. 4. I was born in 1831 and showed that electricity and magnetism are scientifically related. 5. He developed t ...
XIX. Chemistry, High School
... The spring 2013 high school Chemistry test was based on learning standards in the Chemistry content strand of the Massachusetts Science and Technology/Engineering Curriculum Framework (2006). These learning standards appear on pages 69–73 of the Framework. The Science and Technology/Engineering Curr ...
... The spring 2013 high school Chemistry test was based on learning standards in the Chemistry content strand of the Massachusetts Science and Technology/Engineering Curriculum Framework (2006). These learning standards appear on pages 69–73 of the Framework. The Science and Technology/Engineering Curr ...
Atomic Theory and Isotopes powerpoint
... bound together. The atoms themselves are not changed in a chemical reaction. Copyright©2000 by Houghton Mifflin Company. All rights reserved. ...
... bound together. The atoms themselves are not changed in a chemical reaction. Copyright©2000 by Houghton Mifflin Company. All rights reserved. ...
Materials Required
... as the students take their seats. As class begins a bag of candy for the activity will be pulled out and the students will be asked what the bag contains while emphasizing what is written on the board. For today this candy will be subatomic particles. Stimulating the recall of prerequisite learning: ...
... as the students take their seats. As class begins a bag of candy for the activity will be pulled out and the students will be asked what the bag contains while emphasizing what is written on the board. For today this candy will be subatomic particles. Stimulating the recall of prerequisite learning: ...