A an electron and an alpha particle B an electron and a proton C a
... empty space ® electrons exist in orbitals outside the nucleus B the atom is a hard sphere ® electrons exist in orbitals outside the nucleus ® most of the atom is empty space C most of the atom is empty space ® electrons exist in orbitals outside the nucleus ® the atom is a hard sphere D most of the ...
... empty space ® electrons exist in orbitals outside the nucleus B the atom is a hard sphere ® electrons exist in orbitals outside the nucleus ® most of the atom is empty space C most of the atom is empty space ® electrons exist in orbitals outside the nucleus ® the atom is a hard sphere D most of the ...
Electrical Forces
... Gravitational force is always attractive. Electric force can be either attractive or repulsive. Forces have both magnitude and direction. It is harder to collect a large electric charge of either sign than a large mass of matter. Thus, gravity is more significant on a large scale (cosmic size), whil ...
... Gravitational force is always attractive. Electric force can be either attractive or repulsive. Forces have both magnitude and direction. It is harder to collect a large electric charge of either sign than a large mass of matter. Thus, gravity is more significant on a large scale (cosmic size), whil ...
Earth’s Materials - Lower Hudson Regional Information Center
... The color of a mineral can be useful, HOWEVER, it can vary due to slight chemical differences The streak is the color of freshly crushed mineral powder and is usually constant. ...
... The color of a mineral can be useful, HOWEVER, it can vary due to slight chemical differences The streak is the color of freshly crushed mineral powder and is usually constant. ...
Balancing and Predicting Chemical Reactions:
... metals are commonly referred to as the “coinage metals”. Why would these metals be chosen over more active metals for use in coins? Why do you think some more active metals, such as zinc or nickel, are sometimes used in coins? ...
... metals are commonly referred to as the “coinage metals”. Why would these metals be chosen over more active metals for use in coins? Why do you think some more active metals, such as zinc or nickel, are sometimes used in coins? ...
Lesson Plan - cloudfront.net
... Trying to measure e-, idea of photons being necessary for us to “see/measure” something How do photons interact with matter: photons can have momentum because they have energy. What happens when something with momentum hits something else? Demonstrate momentum interactions with bowling balls. What d ...
... Trying to measure e-, idea of photons being necessary for us to “see/measure” something How do photons interact with matter: photons can have momentum because they have energy. What happens when something with momentum hits something else? Demonstrate momentum interactions with bowling balls. What d ...
Chem 173: Final Exam Review Short Answer and Problems 1
... Limestone is composed of calcium carbonate (CaCO3) as well as other compounds. In an analysis, a chemist takes a sample of limestone which has a mass of 413 mg and treats it with oxalic acid (H2C 2O4). A chemical reaction occurs between the calcium carbonate and the acid producing calcium oxalate an ...
... Limestone is composed of calcium carbonate (CaCO3) as well as other compounds. In an analysis, a chemist takes a sample of limestone which has a mass of 413 mg and treats it with oxalic acid (H2C 2O4). A chemical reaction occurs between the calcium carbonate and the acid producing calcium oxalate an ...
Stoichiometry
... from the reaction of 0.10 mole of Mg3N2? • How many moles of NH3 would be produced from the reaction of 500. g of Mg3N2? • How many molecules of water would be required to react with 3.64 g of Mg3N2? • What is the maximum number of grams of Mg(OH)2 that can be produced by the reaction of 10.0 g of M ...
... from the reaction of 0.10 mole of Mg3N2? • How many moles of NH3 would be produced from the reaction of 500. g of Mg3N2? • How many molecules of water would be required to react with 3.64 g of Mg3N2? • What is the maximum number of grams of Mg(OH)2 that can be produced by the reaction of 10.0 g of M ...
Chapter 4 PowerPoint - Southeast Online
... 2. All atoms of an element are identical. They have the same mass, volume, and other physical and ...
... 2. All atoms of an element are identical. They have the same mass, volume, and other physical and ...
atm-atomic structure - Discovery Education
... “not cuttable.” It was given this name after Democritus developed his theory of atomism. Based on this theory, atoms were the smallest parts of an element that could not be broken down any further. Atom is defined as a tiny indivisible particle of which the universe is composed.) ...
... “not cuttable.” It was given this name after Democritus developed his theory of atomism. Based on this theory, atoms were the smallest parts of an element that could not be broken down any further. Atom is defined as a tiny indivisible particle of which the universe is composed.) ...
Review for Final Exam - Short Answer and Problems
... Limestone is composed of calcium carbonate (CaCO3) as well as other compounds. In an analysis, a chemist takes a sample of limestone which has a mass of 413 mg and treats it with oxalic acid (H2C2O4). A chemical reaction occurs between the calcium carbonate and the acid producing calcium oxalate and ...
... Limestone is composed of calcium carbonate (CaCO3) as well as other compounds. In an analysis, a chemist takes a sample of limestone which has a mass of 413 mg and treats it with oxalic acid (H2C2O4). A chemical reaction occurs between the calcium carbonate and the acid producing calcium oxalate and ...
Heat capacity - Department of Chemistry and Physics
... - A mirror shatters when dropped and does not reform - It is easy to scramble an egg and difficult to unscramble it - Food dye when dropped into water disperses ...
... - A mirror shatters when dropped and does not reform - It is easy to scramble an egg and difficult to unscramble it - Food dye when dropped into water disperses ...
Ch 4 PPT - mvhs
... • This occurs because the 4s and 3d orbitals are very close in energy. • These anomalies occur in f-block atoms, as well. ...
... • This occurs because the 4s and 3d orbitals are very close in energy. • These anomalies occur in f-block atoms, as well. ...
Document
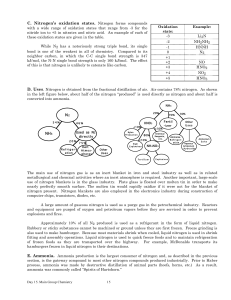
... substance forms from the mixture of two solutions of ionic substances. 2. Acid–base reactions: reactions that involve the transfer of a proton (H+) between reactants 3. Oxidation–reduction reactions: reactions that involve the transfer of electrons between reactants. ...
... substance forms from the mixture of two solutions of ionic substances. 2. Acid–base reactions: reactions that involve the transfer of a proton (H+) between reactants 3. Oxidation–reduction reactions: reactions that involve the transfer of electrons between reactants. ...
CHEM 250Q
... The potter knows that the clay went through a physical change when it became a bowl because the bowl A. ...
... The potter knows that the clay went through a physical change when it became a bowl because the bowl A. ...
Topic_4
... in scientific notation, a mole is 6.02 x 1023 particles. Scientific notation is used to express very small or very large measurements in powers of ten. It expresses quantities by using a number between one and ten, which is then multiplied by ten to a power to give the quantity its proper magnitude. ...
... in scientific notation, a mole is 6.02 x 1023 particles. Scientific notation is used to express very small or very large measurements in powers of ten. It expresses quantities by using a number between one and ten, which is then multiplied by ten to a power to give the quantity its proper magnitude. ...
Introductory Chemistry, 2nd Edition Nivaldo Tro
... 2. All atoms of an element are identical. They have the same mass, volume, and other physical and ...
... 2. All atoms of an element are identical. They have the same mass, volume, and other physical and ...
1.9 M - Thierry Karsenti
... 1. A pure substance: A substance with a definite chemical composition. 2. Atom: the smallest particle of an element that retains the identify and properties of the element and can take part in a chemical change. 3. Atomic number (symbol Z): the number of protons in the nucleus of each atom. 4. Compo ...
... 1. A pure substance: A substance with a definite chemical composition. 2. Atom: the smallest particle of an element that retains the identify and properties of the element and can take part in a chemical change. 3. Atomic number (symbol Z): the number of protons in the nucleus of each atom. 4. Compo ...
C5H12 + 8 O2 → 5 CO2 + 6 H2O
... e.g.: O2, halogens, H2O2, HNO3, Cr2O7–, MnO4– • Reducing agents: Elements or compounds that reduce the other reactant. e.g.: H2, C, metals ...
... e.g.: O2, halogens, H2O2, HNO3, Cr2O7–, MnO4– • Reducing agents: Elements or compounds that reduce the other reactant. e.g.: H2, C, metals ...
2-1 The Nature of Matter
... The number of protons in an atom of an element is the element's atomic number. Carbon has 6 protons, so its atomic number is 6. More than 100 elements are known, but only about two dozen are commonly found in living organisms. ...
... The number of protons in an atom of an element is the element's atomic number. Carbon has 6 protons, so its atomic number is 6. More than 100 elements are known, but only about two dozen are commonly found in living organisms. ...
Ch 3 Chemical Reactions 2013-Sept-08
... Metal Sulfides are black and metal sulfides come from the center of the earth. Sulfides are insoluble in water so they form a black mass in the deep ocean floor cracks. Chemical Reactions are the heart of Chemistry. This chapter is an introduction to symbols and chemical reactions. 3.1 Intro to Chem ...
... Metal Sulfides are black and metal sulfides come from the center of the earth. Sulfides are insoluble in water so they form a black mass in the deep ocean floor cracks. Chemical Reactions are the heart of Chemistry. This chapter is an introduction to symbols and chemical reactions. 3.1 Intro to Chem ...