Name Period
... 6. What does the atomic mass tell about the element above (silver)? 7. In what order did Mendeleev arrange the elements in the periodic table? (LOOK UP in text) 8. What can you predict about an element from its position in the periodic table? (other than atomic mass and atomic number) Vocabulary Fro ...
... 6. What does the atomic mass tell about the element above (silver)? 7. In what order did Mendeleev arrange the elements in the periodic table? (LOOK UP in text) 8. What can you predict about an element from its position in the periodic table? (other than atomic mass and atomic number) Vocabulary Fro ...
Activity - Periodic Table Scavenger Hunt
... 1. Find the element that makes plumbing pipes. Is it a metal, nonmetal or metalloid? 2. Find the element that makes glowing signs, what special group is it in? 3. Find the element that makes milk good for your health. Is it a metal, nonmetal or metalloid? ...
... 1. Find the element that makes plumbing pipes. Is it a metal, nonmetal or metalloid? 2. Find the element that makes glowing signs, what special group is it in? 3. Find the element that makes milk good for your health. Is it a metal, nonmetal or metalloid? ...
alkaline earth metals
... something about how reactive the element is. • Elements in Groups 1 and 17 are especially reactive. • Group 18 (the Noble Gases) are least reactive. ...
... something about how reactive the element is. • Elements in Groups 1 and 17 are especially reactive. • Group 18 (the Noble Gases) are least reactive. ...
PeriodicTableNotes
... the square. (typically has a decimal point with numbers after it) o The _____________ ____________ is the number given to element that represents its place on the periodic table. This number is generally the smaller of the two numbers and is normally at the top of the square. It is also the number o ...
... the square. (typically has a decimal point with numbers after it) o The _____________ ____________ is the number given to element that represents its place on the periodic table. This number is generally the smaller of the two numbers and is normally at the top of the square. It is also the number o ...
Physical properties
... A unique number that identifies how many protons are in one atom of that element. the physical property of shininess or the way a substance reflects light; a dull luster means that the substance is not shiny a physical property of metals that allows them to be hammered into different shapes a physic ...
... A unique number that identifies how many protons are in one atom of that element. the physical property of shininess or the way a substance reflects light; a dull luster means that the substance is not shiny a physical property of metals that allows them to be hammered into different shapes a physic ...
CHMB homework Name © Van Der Sluys, 2004 Periodic Table 1
... 18. Elements in a group have the same number of ________________. 19. Elements across a period have the same number of ____________________. 20. A colored ion is a (s, p, d, or f) block element. 21. As you go down a group the elements generally become (more / less) metallic. 22. The majority of elem ...
... 18. Elements in a group have the same number of ________________. 19. Elements across a period have the same number of ____________________. 20. A colored ion is a (s, p, d, or f) block element. 21. As you go down a group the elements generally become (more / less) metallic. 22. The majority of elem ...
Periodic Table Notes Page
... The elements are listed on the Periodic Table in _____________________ ________________, starting at the upper left corner and then moving from the left to right and top to bottom, just as the words of a paragraph are read. The element’s ___________________ is based on the number of protons in each ...
... The elements are listed on the Periodic Table in _____________________ ________________, starting at the upper left corner and then moving from the left to right and top to bottom, just as the words of a paragraph are read. The element’s ___________________ is based on the number of protons in each ...
Ch. 14 Test Review
... increases (3) groups (2) periods transition metals ionization energy atomic # noble gases representative electronegativity The periodic table organizes the elements into vertical ____________ and horizontal ____________ in order of increasing _________________. The table is constructed so that eleme ...
... increases (3) groups (2) periods transition metals ionization energy atomic # noble gases representative electronegativity The periodic table organizes the elements into vertical ____________ and horizontal ____________ in order of increasing _________________. The table is constructed so that eleme ...
Chapter 8 Study Guide
... Period- (7) rows of the periodic table; elements are NOT similar in same period Most of the elements are classified as metals. Properties of elements change as you go across a period. Alkali Metals I- most reactive metals Alkaline Earth Metals II- not as reactive as Alkali Metals Halogens (VII)- mos ...
... Period- (7) rows of the periodic table; elements are NOT similar in same period Most of the elements are classified as metals. Properties of elements change as you go across a period. Alkali Metals I- most reactive metals Alkaline Earth Metals II- not as reactive as Alkali Metals Halogens (VII)- mos ...
Honors Chemistry- Chapter 5 Homework Packet The Periodic Law
... 2) What is the relationship between the electron configuration of an element and the period in which that element appears on the periodic table? ...
... 2) What is the relationship between the electron configuration of an element and the period in which that element appears on the periodic table? ...
Periodic Table Notes The Periodic Table Use Resource #1
... Use Resource #2 to color the periodic table. 1. Color code each section of the periodic table (metalloids, halogens, alkali metals, etc.) a different color. In order to read the information in the table, do not color too darkly. 2. Create a key for the colors you used. Metals, Nonmetals, and Metallo ...
... Use Resource #2 to color the periodic table. 1. Color code each section of the periodic table (metalloids, halogens, alkali metals, etc.) a different color. In order to read the information in the table, do not color too darkly. 2. Create a key for the colors you used. Metals, Nonmetals, and Metallo ...
1. In what order did Mendeleev arrange the elements in his periodic
... b) decreasing atomic number c) increasing number of neutrons d) increasing atomic weight 2. What family of elements was unknown when Mendeleev created the periodic table? a) noble gases b) alkali metals c) alkaline earth metals d) halogens 3. Mendeleev predicted the existence of which then unknown e ...
... b) decreasing atomic number c) increasing number of neutrons d) increasing atomic weight 2. What family of elements was unknown when Mendeleev created the periodic table? a) noble gases b) alkali metals c) alkaline earth metals d) halogens 3. Mendeleev predicted the existence of which then unknown e ...
2.2 Periodic Chart
... The Alkaline Earth Family The alkaline earth metal family is less reactive than the alkali metals but alkaline earth metals burn brightly in air, some very colourful (strontium is used in red fireworks). Alkaline earth elements also react with water and are very common elements in earth rocks (calc ...
... The Alkaline Earth Family The alkaline earth metal family is less reactive than the alkali metals but alkaline earth metals burn brightly in air, some very colourful (strontium is used in red fireworks). Alkaline earth elements also react with water and are very common elements in earth rocks (calc ...
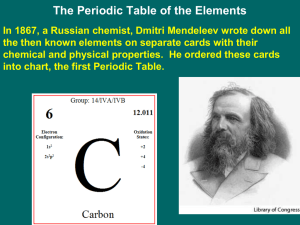
The Periodic Table
... Henry Moseley changed Mendeleev’s periodic table and put the atoms in order according to increasing atomic number (protons) This fixed the problem. Now all of the elements fell into place ...
... Henry Moseley changed Mendeleev’s periodic table and put the atoms in order according to increasing atomic number (protons) This fixed the problem. Now all of the elements fell into place ...
In the space provided, write the letter of the term or phrase that best
... ______ 5. Nonmetallic elements in Group 17 that react with most metals to form salts are a. alkali metals. b. halogens. c. lanthanides. d. noble gases. ______ 6. The outer-level electron configuration of a neutral alkaline-earth metal atom consists of a. one electron in the s orbital. b. two electro ...
... ______ 5. Nonmetallic elements in Group 17 that react with most metals to form salts are a. alkali metals. b. halogens. c. lanthanides. d. noble gases. ______ 6. The outer-level electron configuration of a neutral alkaline-earth metal atom consists of a. one electron in the s orbital. b. two electro ...
PERIODIC TABLE - WordPress.com
... 2. State three physical properties of metals. 3. What is atomic number? 4. What are the atomic numbers and relative atomic masses of Sodium and Chlorine? 5. What are d-block elements commonly known as? 6. Name three metalloids (semi-metals) from the Periodic Table. 7. Which block (s, p, d, f) does i ...
... 2. State three physical properties of metals. 3. What is atomic number? 4. What are the atomic numbers and relative atomic masses of Sodium and Chlorine? 5. What are d-block elements commonly known as? 6. Name three metalloids (semi-metals) from the Periodic Table. 7. Which block (s, p, d, f) does i ...
Reading the Periodic Table
... On the Outside—Write this! • Matter is made up of extremely small particles called ATOMS • Atoms are the smallest part of an element that has the chemical properties of the element. • Elements are composed of only one type of atom. • A single atom has mass and takes up space ...
... On the Outside—Write this! • Matter is made up of extremely small particles called ATOMS • Atoms are the smallest part of an element that has the chemical properties of the element. • Elements are composed of only one type of atom. • A single atom has mass and takes up space ...
The Periodic Table Chemistry – Leaving Cert Quick Notes
... Davy isolated potassium and sodium from their hydroxide compounds. Henry Moseley used the concept of atomic number to derive another definition for an element: a substance all of those whose atoms have the same atomic number. Dobereiner suggested elements of similar properties could be arranged in g ...
... Davy isolated potassium and sodium from their hydroxide compounds. Henry Moseley used the concept of atomic number to derive another definition for an element: a substance all of those whose atoms have the same atomic number. Dobereiner suggested elements of similar properties could be arranged in g ...
HISTORY OF THE PERIODIC TABLE
... I GREEKS (400 B.C.) – used the words “element” and “atom”. II Antoine Lavoisier (1700’s) – divided the element into 4 Classes III Dobereiner (1800’s) – noted the similar elements often had relative atomic masses A.E. Beguyer de Chancourtois – created a cylindrical table of elements to display the pe ...
... I GREEKS (400 B.C.) – used the words “element” and “atom”. II Antoine Lavoisier (1700’s) – divided the element into 4 Classes III Dobereiner (1800’s) – noted the similar elements often had relative atomic masses A.E. Beguyer de Chancourtois – created a cylindrical table of elements to display the pe ...
Ch 6.1 and 6.2 Review
... Compare and contrast the electron configurations of elements within the same family and elements within the same period. Look particularly at sublevels and energy levels. ...
... Compare and contrast the electron configurations of elements within the same family and elements within the same period. Look particularly at sublevels and energy levels. ...
Chapter 5
... 〉Why do elements within a group of the periodic table have similar chemical properties? 〉The periodic trends in the periodic table are the result of electron arrangement. ...
... 〉Why do elements within a group of the periodic table have similar chemical properties? 〉The periodic trends in the periodic table are the result of electron arrangement. ...
Group 3 element

Group 3 is a group of elements in the periodic table. This group, like other d-block groups, should contain four elements, but it is not agreed what elements belong in the group. Scandium (Sc) and yttrium (Y) are always included, but the other two spaces are usually occupied by lanthanum (La) and actinium (Ac), or by lutetium (Lu) and lawrencium (Lr); less frequently, it is considered the group should be expanded to 32 elements (with all the lanthanides and actinides included) or contracted to contain only scandium and yttrium. The group itself has not acquired a trivial name; however, scandium, yttrium and the lanthanides are sometimes called rare earth metals.Three group 3 elements occur naturally, scandium, yttrium, and either lanthanum or lutetium. Lanthanum continues the trend started by two lighter members in general chemical behavior, while lutetium behaves more similarly to yttrium. This is in accordance with the trend for period 6 transition metals to behave more similarly to their upper periodic table neighbors. This trend is seen from hafnium, which is almost identical chemically to zirconium, to mercury, which is quite distant chemically from cadmium, but still shares with it almost equal atomic size and other similar properties. They all are silvery-white metals under standard conditions. The fourth element, either actinium or lawrencium, has only radioactive isotopes. Actinium, which occurs only in trace amounts, continues the trend in chemical behavior for metals that form tripositive ions with a noble gas configuration; synthetic lawrencium is calculated and partially shown to be more similar to lutetium and yttrium. So far, no experiments have been conducted to synthesize any element that could be the next group 3 element. Unbiunium (Ubu), which could be considered a group 3 element if preceded by lanthanum and actinium, might be synthesized in the near future, it being only three spaces away from the current heaviest element known, ununoctium.