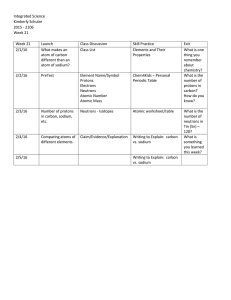
Week 21 Lessons - Highline Public Schools
... Bohr Diagram is located at the end of More about orbitals page. (If you want, you can search Lewis dot structure.) Note: We will work on more understanding over this unit. Exit Ticket: i. The number of protons in carbon is . . . ii. I know this because . . . HS-PS1-1. Use the periodic table as a m ...
... Bohr Diagram is located at the end of More about orbitals page. (If you want, you can search Lewis dot structure.) Note: We will work on more understanding over this unit. Exit Ticket: i. The number of protons in carbon is . . . ii. I know this because . . . HS-PS1-1. Use the periodic table as a m ...
the atomic theory
... 3. A fictitious element is composed of isotopes A and B with masses of 61.9887 and 64.9486 amu, respectfully. The atomic mass of the element is 64.52 amu. What can you conclude about the natural abundances of the two isotopes? Briefly explain your answer. ...
... 3. A fictitious element is composed of isotopes A and B with masses of 61.9887 and 64.9486 amu, respectfully. The atomic mass of the element is 64.52 amu. What can you conclude about the natural abundances of the two isotopes? Briefly explain your answer. ...
Periodic Table
... Known properties were: melting point, density, color, atomic mass, # of chemical bonds an element can form. Atomic mass is the average mass of one atom of that element. ...
... Known properties were: melting point, density, color, atomic mass, # of chemical bonds an element can form. Atomic mass is the average mass of one atom of that element. ...
power point
... grouped as metals, nonmetals, and semimetals. Metals make up most of the periodic table and are located in the center and at the left of the table. With the exception of hydrogen, nonmetals are on the right side, and semimetals are located between the metals and nonmetals. ...
... grouped as metals, nonmetals, and semimetals. Metals make up most of the periodic table and are located in the center and at the left of the table. With the exception of hydrogen, nonmetals are on the right side, and semimetals are located between the metals and nonmetals. ...
The Periodic Table - Brookwood High School
... Most group A and all group B elements are metals Group 1A elements (except H) are alkali metals Group 2A elements are alkaline earth metals; both are chemically reactive, alkali the more reactive Elements from group B (lanthanide series) are used as phosphors, substances that emit light when struck ...
... Most group A and all group B elements are metals Group 1A elements (except H) are alkali metals Group 2A elements are alkaline earth metals; both are chemically reactive, alkali the more reactive Elements from group B (lanthanide series) are used as phosphors, substances that emit light when struck ...
Year 10 Chemistry Exam June 2012
... The reactivity. The number of outer-shell electrons in each atom. The size of atoms. The melting point. ...
... The reactivity. The number of outer-shell electrons in each atom. The size of atoms. The melting point. ...
File
... Periods and Blocks of the Periodic Table Periods – horizontal rows Corresponds to highest principal quantum number Groups/Families – vertical columns; these elements ...
... Periods and Blocks of the Periodic Table Periods – horizontal rows Corresponds to highest principal quantum number Groups/Families – vertical columns; these elements ...
Chemistry 4.1 Atomic structure and the periodic table NEED TO
... are non-metals consist of molecules which are made up of pairs of atoms react with metals to form ionic compounds in which the halide ion carries a charge of -1 form molecular compounds with other non-metallic elements. In Group 7, the further down the group an element is, the higher its rel ...
... are non-metals consist of molecules which are made up of pairs of atoms react with metals to form ionic compounds in which the halide ion carries a charge of -1 form molecular compounds with other non-metallic elements. In Group 7, the further down the group an element is, the higher its rel ...
Review Packet - Old Saybrook Public Schools
... The scientist who observed a pattern of properties that repeated every eight elements was ...
... The scientist who observed a pattern of properties that repeated every eight elements was ...
Chapter 5 - Midway ISD
... Combined with s-block they are called main-group elements Contains metals, non-metals, and metalloids, thus wide range of properties Group 17 – halogens ...
... Combined with s-block they are called main-group elements Contains metals, non-metals, and metalloids, thus wide range of properties Group 17 – halogens ...
Organic Functional Groups: Halocarbons
... • The halogens are in the second column from the right. • Astatine (At) is not included: it is unstable and extremely rare. • Halogens each have seven valence electrons – three more than carbon. • Halogens are very hungry to gain an 8th electron. When they do, the eight electrons collectively drop i ...
... • The halogens are in the second column from the right. • Astatine (At) is not included: it is unstable and extremely rare. • Halogens each have seven valence electrons – three more than carbon. • Halogens are very hungry to gain an 8th electron. When they do, the eight electrons collectively drop i ...
Unit 3 Test Review – Periodic Table (Yes, this is worth a grade!) Fill
... _____ 19. best conductor of heat and electricity. _____ 20. has a completely filled outer energy level _____ 21. used in advertising signs _____ 22. forms table salt when combined with chlorine _____ 23. used for coins, fine eating utensils and jewelry _____ 24. has 1 valance electron _____ 25. unre ...
... _____ 19. best conductor of heat and electricity. _____ 20. has a completely filled outer energy level _____ 21. used in advertising signs _____ 22. forms table salt when combined with chlorine _____ 23. used for coins, fine eating utensils and jewelry _____ 24. has 1 valance electron _____ 25. unre ...
The Periodic Table
... The noble gases (inert gases) are the elements in Group 8A of the periodic table. S & P sublevels are completely filled with electrons. Due to filled outer energy level, noble gases do not readily react with other elements. ...
... The noble gases (inert gases) are the elements in Group 8A of the periodic table. S & P sublevels are completely filled with electrons. Due to filled outer energy level, noble gases do not readily react with other elements. ...
Period - scienceathylands
... • They are neutralised by acids to form salt and water. e.g. MgO(s) + 2HCl(aq) MgCl2(aq) + H2O(l) or Ca(OH)2(s) + 2HCl(aq) CaCl2(aq) + 2H2O(l) As the products of these reactions are soluble you will see the solid oxides or hydroxides dissolve ...
... • They are neutralised by acids to form salt and water. e.g. MgO(s) + 2HCl(aq) MgCl2(aq) + H2O(l) or Ca(OH)2(s) + 2HCl(aq) CaCl2(aq) + 2H2O(l) As the products of these reactions are soluble you will see the solid oxides or hydroxides dissolve ...
Develo ment of the Atomic Theo John Dalton - (English 1766
... When Mendeleev looked over his periodic table, he did notice that some of the elements appeared to be misplaced in terms of their properties. He assumed that their atomic mass was just calculated incorrectly. 50 yrs. later a British scientist - Henry Moseley discovered the atomic numbers of the elem ...
... When Mendeleev looked over his periodic table, he did notice that some of the elements appeared to be misplaced in terms of their properties. He assumed that their atomic mass was just calculated incorrectly. 50 yrs. later a British scientist - Henry Moseley discovered the atomic numbers of the elem ...
Ch. 6 - The Periodic Table
... Relatively soft, but harder than alkali metals. Two valence electrons. Although not as reactive as alkali metals, still very reactive and not found in nature in the elemental state. ◦ Obtained in the pure form through electrolysis of their fused salts. ...
... Relatively soft, but harder than alkali metals. Two valence electrons. Although not as reactive as alkali metals, still very reactive and not found in nature in the elemental state. ◦ Obtained in the pure form through electrolysis of their fused salts. ...
4-2 A Guided Tour of the Periodic Table
... An amu is equal to one-twelfth of the mass of a carbon-12 atom. This isotope of carbon has six protons and six neutrons, so individual protons and neutrons must each have a mass of about 1.0 amu, because electrons contribute very little mass. ...
... An amu is equal to one-twelfth of the mass of a carbon-12 atom. This isotope of carbon has six protons and six neutrons, so individual protons and neutrons must each have a mass of about 1.0 amu, because electrons contribute very little mass. ...
Chemistry 4.2
... current. • Most nonmetals are gases at room temperature. • A few nonmetals are solids, such as sulfur and phosphorus. • One nonmetal, bromine, is a dark-red liquid. ...
... current. • Most nonmetals are gases at room temperature. • A few nonmetals are solids, such as sulfur and phosphorus. • One nonmetal, bromine, is a dark-red liquid. ...
CHEM121 Lecture Ch2
... – primary substances from which all other things are built. – Cannot be broken down into simpler substances ...
... – primary substances from which all other things are built. – Cannot be broken down into simpler substances ...
questions on periodic classification of elements
... of the bond formed between them? To which period of the Modern periodic table do these two elements belong? 11. The elements Li, Be, B , C ,N, O ,F and Ne are present in the second period . How many valence electrons do these elements have? Classify them as metals and non metals. 12. An atom ‘X’ has ...
... of the bond formed between them? To which period of the Modern periodic table do these two elements belong? 11. The elements Li, Be, B , C ,N, O ,F and Ne are present in the second period . How many valence electrons do these elements have? Classify them as metals and non metals. 12. An atom ‘X’ has ...
The History of the Modern Periodic Table
... achievement, problems arose when new elements were discovered and more accurate atomic weights determined. By looking at our modern periodic table, can you identify what problems might have caused chemists a headache? Ar and K Co and Ni Te and I Th and Pa ...
... achievement, problems arose when new elements were discovered and more accurate atomic weights determined. By looking at our modern periodic table, can you identify what problems might have caused chemists a headache? Ar and K Co and Ni Te and I Th and Pa ...
Power point notes - Social Circle City Schools
... metals and non-metals. They are solids that can be shiny or dull. They conduct heat and electricity better than nonmetals but not as well as metals. They are ductile and ...
... metals and non-metals. They are solids that can be shiny or dull. They conduct heat and electricity better than nonmetals but not as well as metals. They are ductile and ...
Slide 1
... Hydrogen has smallest atomic mass Mendeleev began to notice more patterns when he arranged the elements by their atomic mass ...
... Hydrogen has smallest atomic mass Mendeleev began to notice more patterns when he arranged the elements by their atomic mass ...
3 Periodic Trends
... Match each of the four trends listed to its description by writing the correct letter of the description next to the trend’s name. Trends _____ Ionic Radius _____ Ionization Energy ...
... Match each of the four trends listed to its description by writing the correct letter of the description next to the trend’s name. Trends _____ Ionic Radius _____ Ionization Energy ...
Period 2 element
The period 2 elements are the chemical elements in the second row (or period) of the periodic table. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. This situation can be explained by modern theories of atomic structure. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the 2s and 2p orbitals. Period 2 elements obey the octet rule in that they need eight electrons to complete their valence shell. The maximum number of electrons that these elements can accommodate is ten, two in the 1s orbital, two in the 2s orbital and six in the 2p orbital. All of the elements in the period can form diatomic molecules except beryllium and neon.























