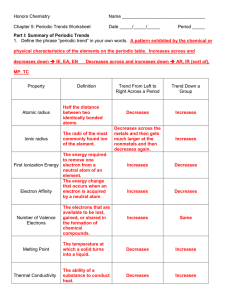
Honors Chemistry
... The easier it is to lose an electron the smaller the amount of energy needed to remove it (smaller ionization energy). 2. What happens to the amount of positive charge in the nucleus as you go from left to right across a period? The amount of positive charge increases as the number of protons increa ...
... The easier it is to lose an electron the smaller the amount of energy needed to remove it (smaller ionization energy). 2. What happens to the amount of positive charge in the nucleus as you go from left to right across a period? The amount of positive charge increases as the number of protons increa ...
AP CHEMISTRY Periodic Trends Worksheet
... The easier it is to lose an electron the smaller the amount of energy needed to remove it (smaller ionization energy). 2. What happens to the amount of positive charge in the nucleus as you go from left to right across a period? The amount of positive charge increases as the number of protons increa ...
... The easier it is to lose an electron the smaller the amount of energy needed to remove it (smaller ionization energy). 2. What happens to the amount of positive charge in the nucleus as you go from left to right across a period? The amount of positive charge increases as the number of protons increa ...
Section 2 Electron Configuration and the Periodic
... • In many compounds, the negative charge of the valence electrons is concentrated closer to one atom than to another. • Electronegativity is a measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound. • Electronegativities tend to increase acros ...
... • In many compounds, the negative charge of the valence electrons is concentrated closer to one atom than to another. • Electronegativity is a measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound. • Electronegativities tend to increase acros ...
Learning Guide 3
... 4. As you go down a group the elements may change from nonmetal metalloid metal. 5. The metallic characteristics increase as you go down a group. 6. As you go left to right metallic characteristics ...
... 4. As you go down a group the elements may change from nonmetal metalloid metal. 5. The metallic characteristics increase as you go down a group. 6. As you go left to right metallic characteristics ...
Review of Electronegativity
... Atomic radius – half the internuclear distance between neighboring atoms of the same element in a metal (metallic radius) or in a molecule (covalent radius). Ionic radius – typically determined by assigning the radius of O2- (in an Oh hole of a solid oxide crystal) as 1.40 Å and subsequently determi ...
... Atomic radius – half the internuclear distance between neighboring atoms of the same element in a metal (metallic radius) or in a molecule (covalent radius). Ionic radius – typically determined by assigning the radius of O2- (in an Oh hole of a solid oxide crystal) as 1.40 Å and subsequently determi ...
Oxidation Numbers, Begin Exam Review
... – Elaborate: Exam Review Packet – Informal assessment as students answer questions – Formal assessment collecting responses ...
... – Elaborate: Exam Review Packet – Informal assessment as students answer questions – Formal assessment collecting responses ...
It gets harder to take them from the atoms towards the right
... b. or this can be done by losing electrons, and thereby stripping off 1 or 2 outer electrons to reveal a full shell underneath (2) s1 would lose an electron, s2 would lose 2 electrons….. and there are full p6 shells underneath 3. When elements react, they are exchanging or sharing electrons to try a ...
... b. or this can be done by losing electrons, and thereby stripping off 1 or 2 outer electrons to reveal a full shell underneath (2) s1 would lose an electron, s2 would lose 2 electrons….. and there are full p6 shells underneath 3. When elements react, they are exchanging or sharing electrons to try a ...
Document
... make a second line to represent Group 2 (Alkaline Earth Metals). Use a periodic table to determine which elements are members of Group 1 and which elements are members of Group 2. 4. What happens to the ionization energy as one goes down a group (use the term “ionization energy” in the response)? ...
... make a second line to represent Group 2 (Alkaline Earth Metals). Use a periodic table to determine which elements are members of Group 1 and which elements are members of Group 2. 4. What happens to the ionization energy as one goes down a group (use the term “ionization energy” in the response)? ...
Chemistry A- Periodic Table Packet
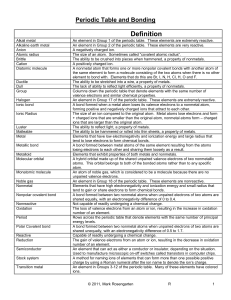
... members touch the zigzag line are called metalloids because they have both metallic and nonmetallic properties. NEW: The table is also arranged in vertical columns called “groups” or “families” and horizontal rows called “periods.” Each arrangement is significant. Groups (vertical, “up and down”) – ...
... members touch the zigzag line are called metalloids because they have both metallic and nonmetallic properties. NEW: The table is also arranged in vertical columns called “groups” or “families” and horizontal rows called “periods.” Each arrangement is significant. Groups (vertical, “up and down”) – ...
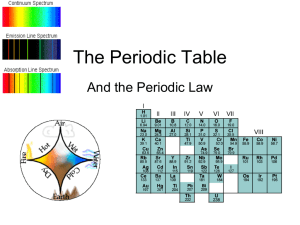
The Organization of the Elements
... number, and therefore organized the table by nuclear charge (or atomic number) rather than atomic weight. Thus Moseley placed argon (atomic number 18) before potassium (atomic number 19) based on their X-ray wavelengths, despite the fact that argon has a greater atomic weight (39.9) than potassium ( ...
... number, and therefore organized the table by nuclear charge (or atomic number) rather than atomic weight. Thus Moseley placed argon (atomic number 18) before potassium (atomic number 19) based on their X-ray wavelengths, despite the fact that argon has a greater atomic weight (39.9) than potassium ( ...
Periodicity Group Project
... will be. If two elements are in the same period, electrons are added to the same energy level, thus the shielding remains the _____________. When elements are in the same group and you go down the periodic table, both Coulombic attraction and Shielding are increasing, but shielding has the greater e ...
... will be. If two elements are in the same period, electrons are added to the same energy level, thus the shielding remains the _____________. When elements are in the same group and you go down the periodic table, both Coulombic attraction and Shielding are increasing, but shielding has the greater e ...
Trends in the Periodic Table
... Linus Pauling, an American chemist (and winner of two Nobel prizes!) came up with the concept of electronegativity in 1932 to help explain the nature of chemical bonds. Today we still measure electronegativities of elements using the Pauling scale. Since fluorine is the most electronegative element ...
... Linus Pauling, an American chemist (and winner of two Nobel prizes!) came up with the concept of electronegativity in 1932 to help explain the nature of chemical bonds. Today we still measure electronegativities of elements using the Pauling scale. Since fluorine is the most electronegative element ...
Honors Chemistry
... The easier it is to lose an electron the smaller the amount of energy needed to remove it (smaller ionization energy). 2. What happens to the amount of positive charge in the nucleus as you go from left to right across a period? The amount of positive charge increases as the number of protons increa ...
... The easier it is to lose an electron the smaller the amount of energy needed to remove it (smaller ionization energy). 2. What happens to the amount of positive charge in the nucleus as you go from left to right across a period? The amount of positive charge increases as the number of protons increa ...
Chapter 7. Periodic Properties of the Elements.
... various ways. The bonding atomic radius, or covalent radius is half the distance in homonuclear diatomic molecules, e.g. H2, Cl2. The C-C distance in diamond gives us the covalent radius of C as 1.54/2 = 0.77 Å. Bond lengths are given quite closely as the sum of covalent radii, e.g. C-Cl = 0.77 + 0. ...
... various ways. The bonding atomic radius, or covalent radius is half the distance in homonuclear diatomic molecules, e.g. H2, Cl2. The C-C distance in diamond gives us the covalent radius of C as 1.54/2 = 0.77 Å. Bond lengths are given quite closely as the sum of covalent radii, e.g. C-Cl = 0.77 + 0. ...
Now
... ‘d block’ elements are called outer transition elements because they contain at most two electrons in their outer shell. The elements for which f sub shells are filling are called the inner transition elements. Based on electronic configuration, all elements are grouped into four categories. They ar ...
... ‘d block’ elements are called outer transition elements because they contain at most two electrons in their outer shell. The elements for which f sub shells are filling are called the inner transition elements. Based on electronic configuration, all elements are grouped into four categories. They ar ...
Inorganic and Physical Chemistry - university of nairobi staff profiles
... 5. Classify each of the following substances as an element or a compound: (a) hydrogen, (b) water, (c) gold, (d) sugar. 6. Classify each of the following as an element, a compound, a homogeneous mixture, or a heterogeneous mixture: (a) seawater, (b) helium gas, (c) sodium chloride (table salt), (d) ...
... 5. Classify each of the following substances as an element or a compound: (a) hydrogen, (b) water, (c) gold, (d) sugar. 6. Classify each of the following as an element, a compound, a homogeneous mixture, or a heterogeneous mixture: (a) seawater, (b) helium gas, (c) sodium chloride (table salt), (d) ...
File - CCHS Chemistry
... It is sometimes possible to force the outer level of an elem in 3rd or higher period to hold more than 8 e-’s ◦ - Extended Octet Noble gas comps are formed this way ...
... It is sometimes possible to force the outer level of an elem in 3rd or higher period to hold more than 8 e-’s ◦ - Extended Octet Noble gas comps are formed this way ...
Chapter 6 the Periodic Table
... Ionic radius When atoms gain electrons, they have a negative charge resulting in a smaller radius. ...
... Ionic radius When atoms gain electrons, they have a negative charge resulting in a smaller radius. ...
The Periodic Table
... Ionic radius When atoms gain electrons, they have a negative charge resulting in a smaller radius. ...
... Ionic radius When atoms gain electrons, they have a negative charge resulting in a smaller radius. ...
Chem Periodicity, Reactivity, Redox 2009 Yingxin
... The more the protons there are in the nucleus, the stronger electrons are attached to the nucleus. =>> Greater I.E. 2. Distance of electron from nucleus (atomic radius) The smaller the distance, the more strongly attached the electron is to the nucleus =>> Greater I.E. 3. Number of electrons between ...
... The more the protons there are in the nucleus, the stronger electrons are attached to the nucleus. =>> Greater I.E. 2. Distance of electron from nucleus (atomic radius) The smaller the distance, the more strongly attached the electron is to the nucleus =>> Greater I.E. 3. Number of electrons between ...
Periodic Table
... because their nuclei do not exert a strong attractive force on electrons. Elements with high ionization energies have high electronegativities due to the strong pull exerted on electrons by the nucleus. In a group, the electronegativity decreases as atomic number increases, as a result of increased ...
... because their nuclei do not exert a strong attractive force on electrons. Elements with high ionization energies have high electronegativities due to the strong pull exerted on electrons by the nucleus. In a group, the electronegativity decreases as atomic number increases, as a result of increased ...
Period 3 element
A period 3 element is one of the chemical elements in the third row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when the periodic table skips a row and a chemical behaviour begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block. Note that there is a 3d orbital, but it is not filled until Period 4, such giving the period table its characteristic shape of ""two rows at a time"". All of the period 3 elements occur in nature and have at least one stable isotope.