11/13 atoms powerpoint
... Most of the particles passed right through A few particles were deflected VERY FEW were greatly deflected ...
... Most of the particles passed right through A few particles were deflected VERY FEW were greatly deflected ...
1 - College of Arts and Sciences
... average of the isotopic masses of an element’s naturally occurring isotopes. ...
... average of the isotopic masses of an element’s naturally occurring isotopes. ...
1 - College of Arts and Sciences
... average of the isotopic masses of an element’s naturally occurring isotopes. ...
... average of the isotopic masses of an element’s naturally occurring isotopes. ...
Name___________________________________ Physical
... C) The proton was discovered by Thomson in 1880. D) Canal rays were found to be made of protons, electrons, and neutrons. E) The neutron was discovered by Chadwick in 1932. ...
... C) The proton was discovered by Thomson in 1880. D) Canal rays were found to be made of protons, electrons, and neutrons. E) The neutron was discovered by Chadwick in 1932. ...
Name Date: __ ______ Chemistry Semester I Final Exam Review
... characteristics of metals, and nonmetals, balancing nuclear reactions, half-life problems, Identifying a pure substance, homogenous mixture, heterogenous mixture, element, and compound ...
... characteristics of metals, and nonmetals, balancing nuclear reactions, half-life problems, Identifying a pure substance, homogenous mixture, heterogenous mixture, element, and compound ...
Cobalt isotopes in industry 60Co is used to irradiate food sources as
... protium (1H). proton – an elementary particle having a rest mass of about 1.673 × 10–27 kg, slightly less than that of a neutron, and a positive electric charge equal and opposite to that of the electron. The number of protons in the nucleus of an atom is the atomic number. radioactive decay – the p ...
... protium (1H). proton – an elementary particle having a rest mass of about 1.673 × 10–27 kg, slightly less than that of a neutron, and a positive electric charge equal and opposite to that of the electron. The number of protons in the nucleus of an atom is the atomic number. radioactive decay – the p ...
Atomic Structure and Isotopes
... Dalton’s Atomic Theory: > All elements are composed of atoms. > Atoms of the same elements are the same. > Atoms of different elements can combine together to make compounds. > Chemical reactions occur when atoms are separated, joined, or rearranged. ...
... Dalton’s Atomic Theory: > All elements are composed of atoms. > Atoms of the same elements are the same. > Atoms of different elements can combine together to make compounds. > Chemical reactions occur when atoms are separated, joined, or rearranged. ...
Trends in the Periodic Table
... 1. __________________ An atom of this element has 1 proton and 1 electron 2. __________________ An atom of this element has 8 protons 3. __________________ An atom of this element has 10 more protons than an atom of oxygen 4. __________________ This element has an atomic number of 12 5. ____________ ...
... 1. __________________ An atom of this element has 1 proton and 1 electron 2. __________________ An atom of this element has 8 protons 3. __________________ An atom of this element has 10 more protons than an atom of oxygen 4. __________________ This element has an atomic number of 12 5. ____________ ...
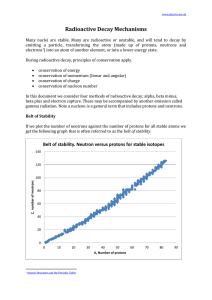
Radioactive Decay Mechanisms
... Radioactive Decay Mechanisms Many nuclei are stable. Many are radioactive or unstable, and will tend to decay by emitting a particle, transforming the atom (made up of protons, neutrons and electrons1) into an atom of another element, or into a lower energy state. During radioactive decay, principle ...
... Radioactive Decay Mechanisms Many nuclei are stable. Many are radioactive or unstable, and will tend to decay by emitting a particle, transforming the atom (made up of protons, neutrons and electrons1) into an atom of another element, or into a lower energy state. During radioactive decay, principle ...
Atoms & Mass Spectrometry
... Nuclei that contain too many or too few neutrons are unstable (radioactive) and change to form more stable nuclei by giving off radiation. ...
... Nuclei that contain too many or too few neutrons are unstable (radioactive) and change to form more stable nuclei by giving off radiation. ...
PHY–309 L. Solutions for homework set # 10. Textbook question Q
... This wikipedia page shows a diagram of a color CRT tube. In any CRT tube — a TV, a monitor, an oscilloscope, or an X-ray tube — a beam of electrons hits the anode (a screen, or just a piece of metal) at high speed. When the atoms in the anode are hit by fast electrons, sometimes the inner electrons ...
... This wikipedia page shows a diagram of a color CRT tube. In any CRT tube — a TV, a monitor, an oscilloscope, or an X-ray tube — a beam of electrons hits the anode (a screen, or just a piece of metal) at high speed. When the atoms in the anode are hit by fast electrons, sometimes the inner electrons ...
Atoms and Isotopes
... They are atoms of the same element that have different Number of Neutrons but must have the same number of Protons. ...
... They are atoms of the same element that have different Number of Neutrons but must have the same number of Protons. ...
SNC 1D chem chpt2
... of protons but a different number of neutrons. Since each isotope has a unique mass number, you can specify an isotope of an element by placing its mass number after the name of the element. EG. Hygrogen-1 and hydrogen-2 ...
... of protons but a different number of neutrons. Since each isotope has a unique mass number, you can specify an isotope of an element by placing its mass number after the name of the element. EG. Hygrogen-1 and hydrogen-2 ...
1000 - Paint Valley Local Schools
... What is form compounds? The alkali metals, found in group 1 of the periodic table are metals that do not occur freely in nature. These metals have only one valence electron in their outer shell. Therefore, they are ready to lose that one electron in ionic bonding with other elements very easily. Thi ...
... What is form compounds? The alkali metals, found in group 1 of the periodic table are metals that do not occur freely in nature. These metals have only one valence electron in their outer shell. Therefore, they are ready to lose that one electron in ionic bonding with other elements very easily. Thi ...
Reading Quiz
... Ions are atoms that have lost or gained electrons. An atom that loses an electron becomes a positive ion (CATION) An atom that gains an electron becomes a negative ion (ANION) ...
... Ions are atoms that have lost or gained electrons. An atom that loses an electron becomes a positive ion (CATION) An atom that gains an electron becomes a negative ion (ANION) ...
Isotopes - Net Texts
... inside the atom. So if a neutron or two is added or removed from the nucleus, then the chemical properties will not change. This means that such an atom would remain in the same place in the periodic table. For example, no matter how many neutrons we add or subtract from a nucleus with 6 protons, th ...
... inside the atom. So if a neutron or two is added or removed from the nucleus, then the chemical properties will not change. This means that such an atom would remain in the same place in the periodic table. For example, no matter how many neutrons we add or subtract from a nucleus with 6 protons, th ...
Chapter 2: Elements are the building blocks of matter
... May conduct electricity Poor conductors of heat ...
... May conduct electricity Poor conductors of heat ...
UC Irvine FOCUS! 5 E Lesson Plan Title: Marble Isotope Lab Grade
... Different atoms of the same element can have different numbers of neutrons. Because the protons and neutrons make up the mass of the atom, different atoms of the same element can have different masses! These are called isotopes. For example: Lithium (Li) has two isotopes. Both isotopes have 3 proton ...
... Different atoms of the same element can have different numbers of neutrons. Because the protons and neutrons make up the mass of the atom, different atoms of the same element can have different masses! These are called isotopes. For example: Lithium (Li) has two isotopes. Both isotopes have 3 proton ...
Getting to Know: Periodic Table
... temperature? Should he group them by color? Which system would be most useful? Mendeleev decided to group the elements by specific chemical properties. After much research, he used the properties and atomic masses of the elements to arrange them in a table. Recording data in tables helps organize a ...
... temperature? Should he group them by color? Which system would be most useful? Mendeleev decided to group the elements by specific chemical properties. After much research, he used the properties and atomic masses of the elements to arrange them in a table. Recording data in tables helps organize a ...
A = Atomic Number
... To find the number of neutrons in an atom, subtract the atomic number from the mass number. # NEUTRONS = MASS NUMBER - ATOMIC NUMBER ...
... To find the number of neutrons in an atom, subtract the atomic number from the mass number. # NEUTRONS = MASS NUMBER - ATOMIC NUMBER ...
File - 7th Grade Science
... • Atomic number – the number of protons in an atom of an element • Atoms of different elements contain different numbers of protons • Neutral atoms have the same number of electrons as protons ...
... • Atomic number – the number of protons in an atom of an element • Atoms of different elements contain different numbers of protons • Neutral atoms have the same number of electrons as protons ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.