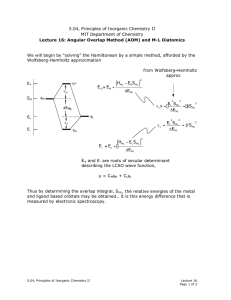
Isometric and unitary phase operators: explaining the Villain transform
... of bosons (photons) introduced by Bialynicki-Birula [2], as explained below. In classical physics a localized or convergent light beam is obtained by superposing plane waves with well-defined phase relations. Since Dirac’s 1927 paper [3], this basic role of the phase has not ceased to motivate the s ...
... of bosons (photons) introduced by Bialynicki-Birula [2], as explained below. In classical physics a localized or convergent light beam is obtained by superposing plane waves with well-defined phase relations. Since Dirac’s 1927 paper [3], this basic role of the phase has not ceased to motivate the s ...
New Bohr model calculates Helium ground state energy
... we have, 2πr = n × mv , mvr = n × 2π , so it is consistent with the equation 1-1 of the Bohr theory. But the Bohr model could not explain about the spin of the electron and the two-electron atoms such as the helium. Because of such problems, the Bohr theory was replaced by the quantum mechanical the ...
... we have, 2πr = n × mv , mvr = n × 2π , so it is consistent with the equation 1-1 of the Bohr theory. But the Bohr model could not explain about the spin of the electron and the two-electron atoms such as the helium. Because of such problems, the Bohr theory was replaced by the quantum mechanical the ...
Document
... representation of a black-body is a pinhole in an otherwise closed container. The radiation is reflected many times within the container and comes to thermal equilibrium with the walls at a temperature T. Radiation leaking out through the pinhole is characteristic of the radiation within the contain ...
... representation of a black-body is a pinhole in an otherwise closed container. The radiation is reflected many times within the container and comes to thermal equilibrium with the walls at a temperature T. Radiation leaking out through the pinhole is characteristic of the radiation within the contain ...
The postulates of Quantum Mechanics
... measured, and they remain constant for a finite time interval, then we can speak about the state of the physical system. It should be noted that there is a difference between a state from the classical point of view and the quantum point of view: a) let us say we have a thermodynamic state of an ide ...
... measured, and they remain constant for a finite time interval, then we can speak about the state of the physical system. It should be noted that there is a difference between a state from the classical point of view and the quantum point of view: a) let us say we have a thermodynamic state of an ide ...
Critical parameters for the heliumlike atoms: A phenomenological
... Kato showed8 that these series have a nonzero radius of convergence. This radius is determined by the distance from the origin to the nearest singularity in the complex plane l * . The study of the radius of convergence, l * , and whether or not this is the same as the critical value of l c has a lo ...
... Kato showed8 that these series have a nonzero radius of convergence. This radius is determined by the distance from the origin to the nearest singularity in the complex plane l * . The study of the radius of convergence, l * , and whether or not this is the same as the critical value of l c has a lo ...
Hund`s multiplicity rule: From atoms to quantum dots
... electronic energy of the system. In the case of He, the gain in the negative electron-nucleus energy contribution for the ...
... electronic energy of the system. In the case of He, the gain in the negative electron-nucleus energy contribution for the ...
Document
... the orbit of Uranus, to predict the general location of the perturbing planet now known as Neptune. Perturbation theory is used to estimate the energies and wave functions for a quantum system described by a potential which is only slightly different than a potential with a known solution. The approa ...
... the orbit of Uranus, to predict the general location of the perturbing planet now known as Neptune. Perturbation theory is used to estimate the energies and wave functions for a quantum system described by a potential which is only slightly different than a potential with a known solution. The approa ...
2. Fundamental principles
... are time independent, and also symmetric with respect to the midpoint of the box. This implies that the expectation value of the position x is equal to L/2. Later we shall see that the expectation values of px (and hence of vx ) are equal to zero for all the stationary states. So there there really ...
... are time independent, and also symmetric with respect to the midpoint of the box. This implies that the expectation value of the position x is equal to L/2. Later we shall see that the expectation values of px (and hence of vx ) are equal to zero for all the stationary states. So there there really ...
Operator methods in quantum mechanics
... This is clearly a discrete transformation. Application of parity twice returns the initial state implying that P̂ 2 = 1. Therefore, the eigenvalues of the parity operation (if such exist) are ±1. A wavefunction will have a defined parity if and only if it is an even or odd function. For example, for ...
... This is clearly a discrete transformation. Application of parity twice returns the initial state implying that P̂ 2 = 1. Therefore, the eigenvalues of the parity operation (if such exist) are ±1. A wavefunction will have a defined parity if and only if it is an even or odd function. For example, for ...
HCC9 Chapter 9 Objectives and Notes
... 1. law of definite proportions/law of constant composition: Discovered by Joseph Proust in the early 1800’s. In a given chemical compound the elements are always combined in the same proportion by mass. a. H2O is always 2.02 g hydrogen for every 16.0 g oxygen, the ratio of the masses never varies. 2 ...
... 1. law of definite proportions/law of constant composition: Discovered by Joseph Proust in the early 1800’s. In a given chemical compound the elements are always combined in the same proportion by mass. a. H2O is always 2.02 g hydrogen for every 16.0 g oxygen, the ratio of the masses never varies. 2 ...