InorgCh2.1
... There are any number of solutions to the Schrödinger equation, each describing an electron in an atomic orbital: 1s, 2s, 2px, 2py…. ...
... There are any number of solutions to the Schrödinger equation, each describing an electron in an atomic orbital: 1s, 2s, 2px, 2py…. ...
Homework 4 Answer Key
... So, the error is about five one-hundredths of a percent. Thus, instead of an ionization potential of –13.6 eV, one would compute –13.6 eV (the error does not appear with only ! takes an excruciatingly sensitive instrument to measure the 3 significant digits). It difference (although the measurement ...
... So, the error is about five one-hundredths of a percent. Thus, instead of an ionization potential of –13.6 eV, one would compute –13.6 eV (the error does not appear with only ! takes an excruciatingly sensitive instrument to measure the 3 significant digits). It difference (although the measurement ...
ptt-file - Parmenides Foundation
... Attempted to design a QM clock to measure time evolution of a physical process. Need to include clock mechanism in the Hamiltonian. The system ‘fuses’ with the clock and changes its behaviour. Also We cannot make ...
... Attempted to design a QM clock to measure time evolution of a physical process. Need to include clock mechanism in the Hamiltonian. The system ‘fuses’ with the clock and changes its behaviour. Also We cannot make ...
QUASICLASSICAL AND QUANTUM SYSTEMS OF ANGULAR FOR QUANTUM-MECHANICAL MODELS WITH SYMMETRIES
... nucleons, systems of quantized angular momenta of rotating extended objects like molecules. Secondly, the other promising area of applications is Schrödinger quantum mechanics of rigid body with its often rather unexpected and very interesting features. Even within this Schrödinger framework the alg ...
... nucleons, systems of quantized angular momenta of rotating extended objects like molecules. Secondly, the other promising area of applications is Schrödinger quantum mechanics of rigid body with its often rather unexpected and very interesting features. Even within this Schrödinger framework the alg ...
Part IV
... where λk= (ħ2k2)/(2mo) (free electron energy) • Eq. (3) is a set of simultaneous, linear, algebraic equations connecting the Ck-G for ...
... where λk= (ħ2k2)/(2mo) (free electron energy) • Eq. (3) is a set of simultaneous, linear, algebraic equations connecting the Ck-G for ...
Slide 1
... • Quantum Theory – describes mathematically the wave properties of electrons and other very small ...
... • Quantum Theory – describes mathematically the wave properties of electrons and other very small ...
Document
... in neat orbits, cannot be correct. Quantum theory describes an electron probability distribution: ...
... in neat orbits, cannot be correct. Quantum theory describes an electron probability distribution: ...
WAVE MECHANICS (Schrödinger, 1926)
... * The energy depends only on the principal quantum number, as in the Bohr model: En = -2.179 X 10-18J /n2 * The orbitals are named by giving the n value followed by a letter symbol for l: l= 0,1, 2, 3, 4, 5, ... s p d f g h ... * All orbitals with the same n are called a “shell”. All orbitals with t ...
... * The energy depends only on the principal quantum number, as in the Bohr model: En = -2.179 X 10-18J /n2 * The orbitals are named by giving the n value followed by a letter symbol for l: l= 0,1, 2, 3, 4, 5, ... s p d f g h ... * All orbitals with the same n are called a “shell”. All orbitals with t ...
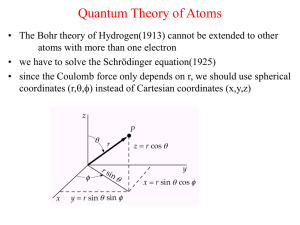
Quantum Theory of Atoms
... • l is the orbital quantum number and the angular momentum of the electron is given by L=[l(l+1)]1/2 ħ • m is the magnetic quantum number and the component of the angular momentum along the z-axis is Lz = m ħ ...
... • l is the orbital quantum number and the angular momentum of the electron is given by L=[l(l+1)]1/2 ħ • m is the magnetic quantum number and the component of the angular momentum along the z-axis is Lz = m ħ ...
Erwin Schrödinger
.jpg?width=300)
Erwin Rudolf Josef Alexander Schrödinger (German: [ˈɛʁviːn ˈʃʁøːdɪŋɐ]; 12 August 1887 – 4 January 1961), sometimes written as Erwin Schrodinger or Erwin Schroedinger, was a Nobel Prize-winning Austrian physicist who developed a number of fundamental results in the field of quantum theory, which formed the basis of wave mechanics: he formulated the wave equation (stationary and time-dependent Schrödinger equation) and revealed the identity of his development of the formalism and matrix mechanics. Schrödinger proposed an original interpretation of the physical meaning of the wave function.In addition, he was the author of many works in various fields of physics: statistical mechanics and thermodynamics, physics of dielectrics, colour theory, electrodynamics, general relativity, and cosmology, and he made several attempts to construct a unified field theory. In his book What Is Life? Schrödinger addressed the problems of genetics, looking at the phenomenon of life from the point of view of physics. He paid great attention to the philosophical aspects of science, ancient and oriental philosophical concepts, ethics, and religion. He also wrote on philosophy and theoretical biology. He is also known for his ""Schrödinger's cat"" thought-experiment.