Reactions in Aqueous Solution
... solutes2, are dispersed uniformly throughout the substance in the greater amount, the solvent3. An aqueous solution4 is a solution in which the solvent is water, whereas in a nonaqueous solution, the solvent is a substance other than water. Familiar examples of nonaqueous solvents are ethyl acetate, ...
... solutes2, are dispersed uniformly throughout the substance in the greater amount, the solvent3. An aqueous solution4 is a solution in which the solvent is water, whereas in a nonaqueous solution, the solvent is a substance other than water. Familiar examples of nonaqueous solvents are ethyl acetate, ...
REACTIONS IN AQUEOUS SOLUTION
... At a young age we learn not to bring electrical devices into the bathtub so as not to electrocute ourselves. That’s a useful lesson because most of the water you encounter in daily life is electrically conducting. Pure water, however, is a very poor conductor of electricity. The conductivity of bath ...
... At a young age we learn not to bring electrical devices into the bathtub so as not to electrocute ourselves. That’s a useful lesson because most of the water you encounter in daily life is electrically conducting. Pure water, however, is a very poor conductor of electricity. The conductivity of bath ...
Chapter - WTPS.org
... • heating wood in the absence of air forms charcoal activated carbon is charcoal used to adsorb other molecules ...
... • heating wood in the absence of air forms charcoal activated carbon is charcoal used to adsorb other molecules ...
Chapter22_LEC
... • heating wood in the absence of air forms charcoal activated carbon is charcoal used to adsorb other molecules ...
... • heating wood in the absence of air forms charcoal activated carbon is charcoal used to adsorb other molecules ...
Secondary Organic Aerosol-Forming Reactions of Glyoxal with
... that these two compounds are responsible for 38% of modeled global SOA, largely via cloud processing (18). The aqueous-phase chemistry of glyoxal is summarized in Scheme 1. Glyoxal takes part in reversible hydration when it enters the condensed phase where it can be oxidized by OH and form oxalic ac ...
... that these two compounds are responsible for 38% of modeled global SOA, largely via cloud processing (18). The aqueous-phase chemistry of glyoxal is summarized in Scheme 1. Glyoxal takes part in reversible hydration when it enters the condensed phase where it can be oxidized by OH and form oxalic ac ...
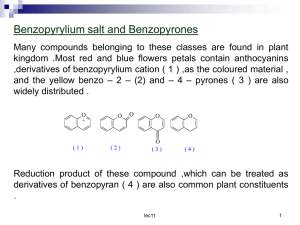
laman web smk raja perempuan, ipoh
... differences in acidity between ethanol and phenol, as well as the differences in basicity between CH3NH2 and C6H5NH2 Candidates should be able to : 1. explain alkanes as saturated aliphatic hydrocarbons 2. explain the construction of the alkane series (straight and branched) and IUPAC nomenclature o ...
... differences in acidity between ethanol and phenol, as well as the differences in basicity between CH3NH2 and C6H5NH2 Candidates should be able to : 1. explain alkanes as saturated aliphatic hydrocarbons 2. explain the construction of the alkane series (straight and branched) and IUPAC nomenclature o ...
Now! - Soojeede.com
... (b) NaOH has Na as a cation, not H (or starts with a cation other than H ) and is therefore not an acid. By writing the dissociation equation we see that NaOH is definitely not an acid. ...
... (b) NaOH has Na as a cation, not H (or starts with a cation other than H ) and is therefore not an acid. By writing the dissociation equation we see that NaOH is definitely not an acid. ...
1 – Introduction
... are increased by the application of ammonium-based fertilizers; these emissions contribute greatly to global climate change as methane is a potent greenhouse gas . Through the increasing use of nitrogen fertilizer, which is added at a rate of 1 billion tons per year presently[64] to the already exis ...
... are increased by the application of ammonium-based fertilizers; these emissions contribute greatly to global climate change as methane is a potent greenhouse gas . Through the increasing use of nitrogen fertilizer, which is added at a rate of 1 billion tons per year presently[64] to the already exis ...
Topic 6 Section C
... A drying agent may not necessarily be a dehydrating agent. For example, anhydrous calcium chloride can dry gases. However, it cannot remove chemically combined water from other compounds. ...
... A drying agent may not necessarily be a dehydrating agent. For example, anhydrous calcium chloride can dry gases. However, it cannot remove chemically combined water from other compounds. ...
Chapter 6
... chemist studying the effect of pollution on spruce trees needs to know, or know where to find, the chemical differences between p‑hydroxybenzoic acid and p‑hydroxyacetophenone, two common phenols found in the needles of spruce trees. Your ability to “think as a chemist” is a product of your experien ...
... chemist studying the effect of pollution on spruce trees needs to know, or know where to find, the chemical differences between p‑hydroxybenzoic acid and p‑hydroxyacetophenone, two common phenols found in the needles of spruce trees. Your ability to “think as a chemist” is a product of your experien ...
Acid Base Equilibrium Diploma Questions
... ____ 37. Acid rain is linked to the leaching of heavy metals and their ions in lakes and rivers. Biomagnification of these metals and ions increases levels of diseases in fish and wildlife. Based on this information, a decision to reduce sulfur dioxide emissions would be a. b. c. d. ...
... ____ 37. Acid rain is linked to the leaching of heavy metals and their ions in lakes and rivers. Biomagnification of these metals and ions increases levels of diseases in fish and wildlife. Based on this information, a decision to reduce sulfur dioxide emissions would be a. b. c. d. ...
Acid rain
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions (low pH). It can have harmful effects on plants, aquatic animals and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids. Governments have made efforts since the 1970s to reduce the release of sulfur dioxide into the atmosphere with positive results. Nitrogen oxides can also be produced naturally by lightning strikes and sulfur dioxide is produced by volcanic eruptions. The chemicals in acid rain can cause paint to peel, corrosion of steel structures such as bridges, and erosion of stone statues.