advanced chemistry may 2011 marking scheme
... reached. Deduct 1 mark if equilibrium concentrations are shown as being equal. (d) The reaction above is involved in the industrial preparation of hydrogen starting from natural gas. Explain the principle of the process giving relevant chemical equations. Natural gas, CH4, (0.5) is oxidized to CO us ...
... reached. Deduct 1 mark if equilibrium concentrations are shown as being equal. (d) The reaction above is involved in the industrial preparation of hydrogen starting from natural gas. Explain the principle of the process giving relevant chemical equations. Natural gas, CH4, (0.5) is oxidized to CO us ...
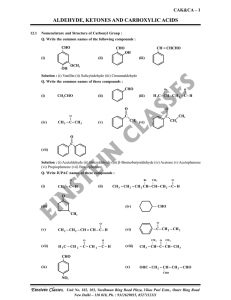
aldehyde, ketones and carboxylic acids
... Solution : Acidic hydrolysis of esters gives directly carboxylic acids while basic hydrolysis gives carboxylates, which on acidification give correspondings carboxylic acids. ...
... Solution : Acidic hydrolysis of esters gives directly carboxylic acids while basic hydrolysis gives carboxylates, which on acidification give correspondings carboxylic acids. ...
CLASS X carbon and its compound
... Ethanol (i) is a colourless and inflammable liquid, (ii) is miscible in water in all proportions, (iii) has a boiling point of 78.2°C and freezing point of – 118°C and (iv) is a bad conductor of electricity. Ethanol reacts with sodium and potassium to form their respective ethoxides and hydrogen gas ...
... Ethanol (i) is a colourless and inflammable liquid, (ii) is miscible in water in all proportions, (iii) has a boiling point of 78.2°C and freezing point of – 118°C and (iv) is a bad conductor of electricity. Ethanol reacts with sodium and potassium to form their respective ethoxides and hydrogen gas ...
Chapter 4 2013
... HF, HI, LiOH, Ca(OH)2, Na2SO4 CH3COO-, NH4+ 2. Classify the following as strong, weak acid or base? HClO4, Sr(OH)2, HClO2, NH3, H3PO4, H2SO4 3. What is the correct formula of the salt formed in the neutralization reaction of hydrochloric acid with calcium ...
... HF, HI, LiOH, Ca(OH)2, Na2SO4 CH3COO-, NH4+ 2. Classify the following as strong, weak acid or base? HClO4, Sr(OH)2, HClO2, NH3, H3PO4, H2SO4 3. What is the correct formula of the salt formed in the neutralization reaction of hydrochloric acid with calcium ...
PRACTICAL ORGANIC CHEMISTRY
... reaction sets in, remove the tube from the flame and wait till the reaction subsides. 4- Heating of the ignition tube is continued gently until no more reaction occurs. 5- Heating is then continued more strongly until the lower part of the tube becomes red, then plunge the hot tube at once, into a b ...
... reaction sets in, remove the tube from the flame and wait till the reaction subsides. 4- Heating of the ignition tube is continued gently until no more reaction occurs. 5- Heating is then continued more strongly until the lower part of the tube becomes red, then plunge the hot tube at once, into a b ...
Werner-type chromium compounds
... the normalmetal salts Thus, a composition in Which thev acido groupsv are cgordinat‘ed with the metal differs radically in chemical properties from a composition inwhich the acido groupsare ...
... the normalmetal salts Thus, a composition in Which thev acido groupsv are cgordinat‘ed with the metal differs radically in chemical properties from a composition inwhich the acido groupsare ...
Acid rain
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions (low pH). It can have harmful effects on plants, aquatic animals and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids. Governments have made efforts since the 1970s to reduce the release of sulfur dioxide into the atmosphere with positive results. Nitrogen oxides can also be produced naturally by lightning strikes and sulfur dioxide is produced by volcanic eruptions. The chemicals in acid rain can cause paint to peel, corrosion of steel structures such as bridges, and erosion of stone statues.