matter crct/final exam review
... 41. Why do atoms share valence electrons or transfer valence electrons? 42. What is the difference between a compound and an element? ...
... 41. Why do atoms share valence electrons or transfer valence electrons? 42. What is the difference between a compound and an element? ...
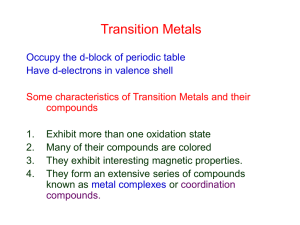
TRANSITION METALS - Pennsylvania State University
... Ligands bonded to metal ions metal complexes or coordination compounds. Coordination number: number of electron donor atoms attached to the metal. Chelates are ligands possessing two or more donor atoms. ...
... Ligands bonded to metal ions metal complexes or coordination compounds. Coordination number: number of electron donor atoms attached to the metal. Chelates are ligands possessing two or more donor atoms. ...
Document
... But the elements of Groups 15, 16, and 17 … and not to mention ions of transition metals can be found in compounds that are not necessarily ionic or binary. ...
... But the elements of Groups 15, 16, and 17 … and not to mention ions of transition metals can be found in compounds that are not necessarily ionic or binary. ...
Atomic Structure(AP MC)
... (A) Atoms contain electrons. (B) Practically all the mass of an atom is contained in its nucleus. (C) Atoms contain protons, neutrons, and electrons. (D) Atoms have a positively charged nucleus surrounded by an electron cloud. (E) No two electrons in one atom can have the same four quantum numbers. ...
... (A) Atoms contain electrons. (B) Practically all the mass of an atom is contained in its nucleus. (C) Atoms contain protons, neutrons, and electrons. (D) Atoms have a positively charged nucleus surrounded by an electron cloud. (E) No two electrons in one atom can have the same four quantum numbers. ...
Provedení, principy činnosti a základy výpočtu pro výměníky tepla
... NO2- nitrite, NO3- nitrate (ion with less oxygens ends with –ite, otherwise –ate) SO3 - - sulfite, SO4 - - sulfate ...
... NO2- nitrite, NO3- nitrate (ion with less oxygens ends with –ite, otherwise –ate) SO3 - - sulfite, SO4 - - sulfate ...
See a sample!
... ducing agents. It can be used to predict reactions between a metal and other metal ions in solution. 4.5 Applications of Oxidation and Reduction— In everyday life, peroxides and hypochlorites are encountered as oxidizing agents. In industry, oxygen gas, chlorine, and chlorine-containing compounds ar ...
... ducing agents. It can be used to predict reactions between a metal and other metal ions in solution. 4.5 Applications of Oxidation and Reduction— In everyday life, peroxides and hypochlorites are encountered as oxidizing agents. In industry, oxygen gas, chlorine, and chlorine-containing compounds ar ...
Organometallics
... methyl radical must donate one electron each to form our metal-ligand bond. Therefore, the methyl group is a one electron donor, not a two electron donor as it is under the ionic formalism. Where did the other electron "go"? It remains on the metal and is counted there. In the covalent method, metal ...
... methyl radical must donate one electron each to form our metal-ligand bond. Therefore, the methyl group is a one electron donor, not a two electron donor as it is under the ionic formalism. Where did the other electron "go"? It remains on the metal and is counted there. In the covalent method, metal ...
IONIC AND COVALENT COMPOUNDS A. Why do Atoms Form
... (3) Molecular formulas = gives type and actual number of atoms in a chemical compound ** exs. H2O molecular & empirical H2O2 molecular HO empirical C6H12O6 molecular CH2O empirical ** note: even though some compounds may have the same empirical formula, their properties are entirely diffe ...
... (3) Molecular formulas = gives type and actual number of atoms in a chemical compound ** exs. H2O molecular & empirical H2O2 molecular HO empirical C6H12O6 molecular CH2O empirical ** note: even though some compounds may have the same empirical formula, their properties are entirely diffe ...
CHEM 120 WEEK 11 LECTURES (INORGANIC WEEK 2) Dr. MD
... Contains only metals, apart from boron. Boron is also the only element which does not form a stable trication (B3+) again will have too high a charge density to be stable. Why do the other elements form tri-cations (M3+ )? Soln. √ Because they have the valence electronic configuration ns2np1 and ...
... Contains only metals, apart from boron. Boron is also the only element which does not form a stable trication (B3+) again will have too high a charge density to be stable. Why do the other elements form tri-cations (M3+ )? Soln. √ Because they have the valence electronic configuration ns2np1 and ...