Temperature
... • We associate the concept of temperature with how hot or cold an objects feels • Our senses provide us with a qualitative indication of temperature • Our senses are unreliable for this purpose • We need a technical definition of temperature ...
... • We associate the concept of temperature with how hot or cold an objects feels • Our senses provide us with a qualitative indication of temperature • Our senses are unreliable for this purpose • We need a technical definition of temperature ...
Page|1 - askIITians
... A calorimeter (from Latin calor, meaning heat) is an object used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. Differential scanning calorimeters, isothermal micro calorimeters, titration calorimeters and accelerated rate ca ...
... A calorimeter (from Latin calor, meaning heat) is an object used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. Differential scanning calorimeters, isothermal micro calorimeters, titration calorimeters and accelerated rate ca ...
Mr Alasdair Ross at Southpointe Academy: Math and Chemistry Pages
... Internal Energy (U) – The internal energy of a system is the total energy contained within a system, partly as kinetic energy and partly as potential energy (See page 218, figure ...
... Internal Energy (U) – The internal energy of a system is the total energy contained within a system, partly as kinetic energy and partly as potential energy (See page 218, figure ...
Force Doubling Paradox of Gravitational Attraction
... Quote "...that one body may act upon another at a distance through a vacuum without the mediation of anything else, by and through which their action and force may be conveyed from one to another, is to me so great an absurdity that I believe no man, who has in philosophic matters a competent facul ...
... Quote "...that one body may act upon another at a distance through a vacuum without the mediation of anything else, by and through which their action and force may be conveyed from one to another, is to me so great an absurdity that I believe no man, who has in philosophic matters a competent facul ...
Powerpoints - University of Pittsburgh
... …So, what are you up to, you frozen whale, you smoked, dried, canned piece of sole…? …Why have you still not sent me your dissertation? …Don't you know that I am one of the 1.5 fellows who would read it with interest and pleasure, you wretched man? I promise you ...
... …So, what are you up to, you frozen whale, you smoked, dried, canned piece of sole…? …Why have you still not sent me your dissertation? …Don't you know that I am one of the 1.5 fellows who would read it with interest and pleasure, you wretched man? I promise you ...
Thermo I
... The amount of heat Q needed for a certain temperature change ΔT is proportional to the temperature change and to the number of moles n of the substance ...
... The amount of heat Q needed for a certain temperature change ΔT is proportional to the temperature change and to the number of moles n of the substance ...
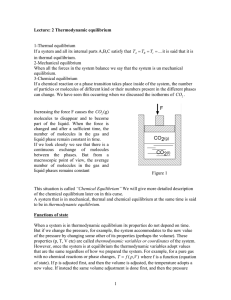
1 Lecture: 2 Thermodynamic equilibrium 1
... constant for a conservative system. If we include all the variables that describe the processes, all systems are conservative. It follows that the energy is always conserved. We consider a system “A” surrounded by the rest of the universe, and we say that the system has a certain amount of energy U ...
... constant for a conservative system. If we include all the variables that describe the processes, all systems are conservative. It follows that the energy is always conserved. We consider a system “A” surrounded by the rest of the universe, and we say that the system has a certain amount of energy U ...
c - Iust personal webpages
... – Associated with forces of attraction or repulsion between objects. ...
... – Associated with forces of attraction or repulsion between objects. ...
The Zeroth Law of Thermodynamics
... This tells us that subsystem A and subsystem C were also in thermal equilibrium. This is always observed to be true. It does not matter what the nature of the systems are or the manner in which they are interrogated. So, Zeroth Law of Thermodynamics If A is in thermal equilibrium with B, and B is in ...
... This tells us that subsystem A and subsystem C were also in thermal equilibrium. This is always observed to be true. It does not matter what the nature of the systems are or the manner in which they are interrogated. So, Zeroth Law of Thermodynamics If A is in thermal equilibrium with B, and B is in ...
Thermodynamics
... A state variable describes the state of a system at time t, but it does not reveal how the system was put into that state. Examples of state variables: pressure, temperature, volume, number of moles, and internal energy. Thermal processes can change the state of a system. We assume that thermal proc ...
... A state variable describes the state of a system at time t, but it does not reveal how the system was put into that state. Examples of state variables: pressure, temperature, volume, number of moles, and internal energy. Thermal processes can change the state of a system. We assume that thermal proc ...
Temperature
... Heat Engines and the Second Law of Thermodynamics. There is an important distinction between heat and work that is not evident from the first law. One manifestation of this distinction is that it is impossible to design a device that, operating in a cyclic fashion, takes in energy by heat and expels ...
... Heat Engines and the Second Law of Thermodynamics. There is an important distinction between heat and work that is not evident from the first law. One manifestation of this distinction is that it is impossible to design a device that, operating in a cyclic fashion, takes in energy by heat and expels ...
20. Heat and the First Law of Thermodynamics
... properties of the system. Experimentally, however, it is observed that the quantity Q - W is the same for all processes. It depends only on the initial and final states and it does not matter at what path is followed to get from one to the other. The quantity Q - W is called the change in the intern ...
... properties of the system. Experimentally, however, it is observed that the quantity Q - W is the same for all processes. It depends only on the initial and final states and it does not matter at what path is followed to get from one to the other. The quantity Q - W is called the change in the intern ...
Thermal radiation
Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. An object with a temperature greater than absolute zero emits thermal radiation. When the temperature of the body is greater than absolute zero, interatomic collisions cause the kinetic energy of the atoms or molecules to change. This results in charge-acceleration and/or dipole oscillation which produces electromagnetic radiation, and the wide spectrum of radiation reflects the wide spectrum of energies and accelerations that occur even at a single temperature.Examples of thermal radiation include the visible light and infrared light emitted by an incandescent light bulb, the infrared radiation emitted by animals and detectable with an infrared camera, and the cosmic microwave background radiation. Thermal radiation is different from thermal convection and thermal conduction—a person near a raging bonfire feels radiant heating from the fire, even if the surrounding air is very cold.Sunlight is part of thermal radiation generated by the hot plasma of the Sun. The Earth also emits thermal radiation, but at a much lower intensity and different spectral distribution (infrared rather than visible) because it is cooler. The Earth's absorption of solar radiation, followed by its outgoing thermal radiation are the two most important processes that determine the temperature and climate of the Earth.If a radiation-emitting object meets the physical characteristics of a black body in thermodynamic equilibrium, the radiation is called blackbody radiation. Planck's law describes the spectrum of blackbody radiation, which depends only on the object's temperature. Wien's displacement law determines the most likely frequency of the emitted radiation, and the Stefan–Boltzmann law gives the radiant intensity.Thermal radiation is one of the fundamental mechanisms of heat transfer.