Acrobat () verson
... If atmospheric pressure is equal to 1.000 atm it can support a column of Hg which is 760.0 mm tall. Suppose a column of Hg is set up where the bath is open to the atmosphere, and the column height of Hg is 760.0 mm with the top of the enclosed column being a vacuum. Next, suppose some diethyl ether ...
... If atmospheric pressure is equal to 1.000 atm it can support a column of Hg which is 760.0 mm tall. Suppose a column of Hg is set up where the bath is open to the atmosphere, and the column height of Hg is 760.0 mm with the top of the enclosed column being a vacuum. Next, suppose some diethyl ether ...
Mole-Mass Conversions
... - Relate counting particles to weighing samples of substances. - Perform mole, particle, mass and volume conversions ...
... - Relate counting particles to weighing samples of substances. - Perform mole, particle, mass and volume conversions ...
Chemical with Petro
... 1. Liquid State: Liquefaction of Gases, Critical constants, Classius-Clayperon Equation; Vapor pressure of Liquids, Salt hydrates, Variation of vaporPressure with temperature. Elementary treatment of vapor pressurecomposition diagrams of Binary liquid mixtures. Azeotropic and Zeotropic mixtures, Fra ...
... 1. Liquid State: Liquefaction of Gases, Critical constants, Classius-Clayperon Equation; Vapor pressure of Liquids, Salt hydrates, Variation of vaporPressure with temperature. Elementary treatment of vapor pressurecomposition diagrams of Binary liquid mixtures. Azeotropic and Zeotropic mixtures, Fra ...
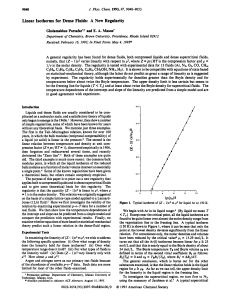
Linear Isotherms for Dense Fluids
... the expression to approach negative infinity. Since TB is proportional to the van der Waals a, this divergence can be attributed to the intermolecularattraction. However, the balance between attraction and repulsion leads to an inflection point and hence to a nearly linear portion in the density ran ...
... the expression to approach negative infinity. Since TB is proportional to the van der Waals a, this divergence can be attributed to the intermolecularattraction. However, the balance between attraction and repulsion leads to an inflection point and hence to a nearly linear portion in the density ran ...
Equilibrium - chemmybear.com
... either equilibrium constant Kp or Kc, for this decomposition reaction. Indicated whether you are calculating Kp or Kc. (c) In order to produce some SbCl5, a 1.00 mole sample of SbCl3 is first placed in an empty 2.00 litre container maintained at a temperature different from 182ºC. At this temperatur ...
... either equilibrium constant Kp or Kc, for this decomposition reaction. Indicated whether you are calculating Kp or Kc. (c) In order to produce some SbCl5, a 1.00 mole sample of SbCl3 is first placed in an empty 2.00 litre container maintained at a temperature different from 182ºC. At this temperatur ...
Chemical Equilibrium is reached when
... reactants and products at equilibrium in terms of a quantity called equilibrium constant. Equilibrium will lie to the right and favor the products, if K >> 1. Equilibrium will lie to the left and favor the reactants, if K << 1. Ways of Expressing Equilibrium Constants: Homogenous equilibrium app ...
... reactants and products at equilibrium in terms of a quantity called equilibrium constant. Equilibrium will lie to the right and favor the products, if K >> 1. Equilibrium will lie to the left and favor the reactants, if K << 1. Ways of Expressing Equilibrium Constants: Homogenous equilibrium app ...
Chapter 1
... translational energies less than or similar to kT , while it is quite improbable that they will have energies much greater than kT . This is our first example of the extremely important Boltzmann distribution. notes-1 ...
... translational energies less than or similar to kT , while it is quite improbable that they will have energies much greater than kT . This is our first example of the extremely important Boltzmann distribution. notes-1 ...
Spontaniety Worked Examples
... Analyze We are given four reactions and asked to predict the sign of ΔS for each. Plan We expect ΔS to be positive if there is an increase in temperature, increase in volume, or increase in number of gas particles. The question states that the temperature is constant, and so we need to concern ourse ...
... Analyze We are given four reactions and asked to predict the sign of ΔS for each. Plan We expect ΔS to be positive if there is an increase in temperature, increase in volume, or increase in number of gas particles. The question states that the temperature is constant, and so we need to concern ourse ...
A matter of Equilibrium
... Although rarely include it in the expression for K, instead of using the partial pressure Px of a component we really should use Px/Pref, so that K is then dimensionless. Pref is the standard pressure. This should be 1 bar, but older books (and some new ones) will have used 1 atm. This can make a di ...
... Although rarely include it in the expression for K, instead of using the partial pressure Px of a component we really should use Px/Pref, so that K is then dimensionless. Pref is the standard pressure. This should be 1 bar, but older books (and some new ones) will have used 1 atm. This can make a di ...
water and aqueous solutions at high pressures and temperatures
... conditions. New PVT-data for water from static measurements are available to 1000°C and 10 kb. Dielectric constants and viscosity have been measured to 550°C and 5 kb. Infra-red and Raman spectra of OD-vibrations of HDO in F120 to 400°C and 5 kb give information about the extent of hydrogen bonded s ...
... conditions. New PVT-data for water from static measurements are available to 1000°C and 10 kb. Dielectric constants and viscosity have been measured to 550°C and 5 kb. Infra-red and Raman spectra of OD-vibrations of HDO in F120 to 400°C and 5 kb give information about the extent of hydrogen bonded s ...
Performance Studies on Sub-cooling of Cryogenic Liquids Used for
... for helium injection application. B. Parameters for analysis and experiments The difference in the Nitrogen partial pressure in helium bubble P GN2,b and the vapour pressure of Liquid Nitrogen Psat(T LN2 ) is the main source of diffusion driven evaporative cooling. Two methods are conceived to incre ...
... for helium injection application. B. Parameters for analysis and experiments The difference in the Nitrogen partial pressure in helium bubble P GN2,b and the vapour pressure of Liquid Nitrogen Psat(T LN2 ) is the main source of diffusion driven evaporative cooling. Two methods are conceived to incre ...
Chromatography and Instrumentation
... The mixture (such as ink ) is placed on the stationary phase (such as filter paper) and the mobile phase (such as water) passes over it. Different parts of the mixture will have different adherence (attraction) to the stationary phase and mobile phase, the components of the mixture which have a bett ...
... The mixture (such as ink ) is placed on the stationary phase (such as filter paper) and the mobile phase (such as water) passes over it. Different parts of the mixture will have different adherence (attraction) to the stationary phase and mobile phase, the components of the mixture which have a bett ...
AP Syllabus 95-96 - Bremen High School District 228
... already missed class does not miss even more class time. Since this is a 2-period class, make up exams and/or labs may require up to two hours after school. Students who miss class for field trips, vacations, or other planned activities not related to illness or emergency should get assignments and ...
... already missed class does not miss even more class time. Since this is a 2-period class, make up exams and/or labs may require up to two hours after school. Students who miss class for field trips, vacations, or other planned activities not related to illness or emergency should get assignments and ...
Liquid-Vapor Equilibria of Llnear Kihara Molecules
... The statistical mechanics of nonspherical molecules have received much attention during the past decade.',z Integral equat i o n ~ ~and " perturbation theories7-I3have now been developed for several nonspherical models. The nonspherical interaction has been typically described by using three differe ...
... The statistical mechanics of nonspherical molecules have received much attention during the past decade.',z Integral equat i o n ~ ~and " perturbation theories7-I3have now been developed for several nonspherical models. The nonspherical interaction has been typically described by using three differe ...
Monte Carlo Calculations for Alcohols and Their Mixtures with
... coexistence curves for methanol, ethanol, propan-1-ol, propan-2-ol, butan-2-ol, 2-methylpropan-2-ol, pentan1-ol, pentane-1,5-diol, and octan-1-ol, and to determine the binary phase diagrams for the mixtures of n-hexane/ methanol and n-hexane/ethanol. It was found that the phase equilibria of the pur ...
... coexistence curves for methanol, ethanol, propan-1-ol, propan-2-ol, butan-2-ol, 2-methylpropan-2-ol, pentan1-ol, pentane-1,5-diol, and octan-1-ol, and to determine the binary phase diagrams for the mixtures of n-hexane/ methanol and n-hexane/ethanol. It was found that the phase equilibria of the pur ...
Shifting Equilibrium
... reaction. This shift decreases the amount of colorless N2O4 gas and increases the amount of brown NO2 gas, as shown in Figure 2.2c. Because less N2O4 gas is present, K is decreased. The change in temperature changes the value of K. For any system in which the forward reaction is an exothermic reacti ...
... reaction. This shift decreases the amount of colorless N2O4 gas and increases the amount of brown NO2 gas, as shown in Figure 2.2c. Because less N2O4 gas is present, K is decreased. The change in temperature changes the value of K. For any system in which the forward reaction is an exothermic reacti ...
GAS LAW PROBLEMS
... 3. Ammonium sulfate, an important fertilizer, can be prepared by the reaction of ammonia with sulfuric acid. Calculate the volume of ammonia needed at 20. degrees C and 25.0 atm to react with 150 kg of sulfuric acid. 2 NH3 + H2SO4 NH4SO4 150 Kg H2SO4 ...
... 3. Ammonium sulfate, an important fertilizer, can be prepared by the reaction of ammonia with sulfuric acid. Calculate the volume of ammonia needed at 20. degrees C and 25.0 atm to react with 150 kg of sulfuric acid. 2 NH3 + H2SO4 NH4SO4 150 Kg H2SO4 ...
Chemistry Review 1 Answer Key
... Since the sand does not dissolve in the water, the mixture can be separated by filtration, by decanting the water, or by evaporating the water. [1 point] 'see explanation below' 17. Base your answer on the accompanying diagram concerning the classification of matter. What type of substance is repres ...
... Since the sand does not dissolve in the water, the mixture can be separated by filtration, by decanting the water, or by evaporating the water. [1 point] 'see explanation below' 17. Base your answer on the accompanying diagram concerning the classification of matter. What type of substance is repres ...
chemistry
... A limiting reactant in a reaction is the species supplied in an amount smaller than that required by the stoichiometric relation between the reactants. A limiting reactant is like a part in an automobile factory, if there are 1000 headlights and 600 car bodies, then the maximum member of automobile ...
... A limiting reactant in a reaction is the species supplied in an amount smaller than that required by the stoichiometric relation between the reactants. A limiting reactant is like a part in an automobile factory, if there are 1000 headlights and 600 car bodies, then the maximum member of automobile ...