
Document
... • Designability. By combining different anions with cations, it is possible to generate a huge number of different ionic liquids, each with their own specific solvent properties. Some ionic liquids are water soluble, others are not. Some dissolve typical organic solvents, other are not. • They can b ...
... • Designability. By combining different anions with cations, it is possible to generate a huge number of different ionic liquids, each with their own specific solvent properties. Some ionic liquids are water soluble, others are not. Some dissolve typical organic solvents, other are not. • They can b ...
reactions
... 2. Preparation of the YBCO bulk superconducting samples. The works in this part of project were focused on the preparation of YBCO bulk superconductors: 1st group – the sintered under varied conditions samples; and 2nd group – the melt-textured under special temperature profiles samples. All samples ...
... 2. Preparation of the YBCO bulk superconducting samples. The works in this part of project were focused on the preparation of YBCO bulk superconductors: 1st group – the sintered under varied conditions samples; and 2nd group – the melt-textured under special temperature profiles samples. All samples ...
PDF 380 KB
... principle. However, in the gauche conformation, there is no center of inversion, and the mutual exclusion rule is no longer applicable. Hence, emergence of this infrared active mode in the Raman spectra at 5 GPa indicates that EG is stabilizing to gauche conformation.21 Above 7 GPa, this mode shows ...
... principle. However, in the gauche conformation, there is no center of inversion, and the mutual exclusion rule is no longer applicable. Hence, emergence of this infrared active mode in the Raman spectra at 5 GPa indicates that EG is stabilizing to gauche conformation.21 Above 7 GPa, this mode shows ...
Equilibrium Reversible Reactions
... Are these the products or reactants? _____________________________________ In graph two which components start with a concentration of 0mol/L? _____________ Are these the products or reactants? _____________________________________ This shows that these two graphs represent the same chemical reactio ...
... Are these the products or reactants? _____________________________________ In graph two which components start with a concentration of 0mol/L? _____________ Are these the products or reactants? _____________________________________ This shows that these two graphs represent the same chemical reactio ...
Equilibrium - pedagogics.ca
... In this case, increasing the temperature causes the position of equilibrium to be shifted to the left, i.e. there is less ammonia present at equilibrium at the higher temperature. The effect of a temperature change on a system at equilibrium can be now considered in terms of Le Chatelier’s principle ...
... In this case, increasing the temperature causes the position of equilibrium to be shifted to the left, i.e. there is less ammonia present at equilibrium at the higher temperature. The effect of a temperature change on a system at equilibrium can be now considered in terms of Le Chatelier’s principle ...
Slide 1
... • Raising the temperature of this system requires the addition of heat, which shifts the equilibrium to the left and reduces the concentration of hydrogen chloride. • Thus, the value of Keq decreases. • Lowering the temperature of the system means that heat is removed, so the equilibrium relieves th ...
... • Raising the temperature of this system requires the addition of heat, which shifts the equilibrium to the left and reduces the concentration of hydrogen chloride. • Thus, the value of Keq decreases. • Lowering the temperature of the system means that heat is removed, so the equilibrium relieves th ...
C143
... that nucleation can begin at radii smaller than those predicted by the nucleation criteria using a static contact angle. Tehvar et al. [6] studied the changes in wetting characteristics of refrigerant R-113 caused by a porous plasma sprayed coating and its influence on the pool boiling characteristi ...
... that nucleation can begin at radii smaller than those predicted by the nucleation criteria using a static contact angle. Tehvar et al. [6] studied the changes in wetting characteristics of refrigerant R-113 caused by a porous plasma sprayed coating and its influence on the pool boiling characteristi ...
22017Stoichiometry
... We can write mole ratios that relate any two substances involved in the reaction. For example: ...
... We can write mole ratios that relate any two substances involved in the reaction. For example: ...
102MSJc14 - Louisiana Tech University
... What would be the Kp of this system if R = 0.0821 liter-atm/mole K? 1. Check to make sure the equation is balanced 2. Calculate n n = Total gas moles on the right - Total gas moles on the left n=2-2=0 3. Calculate T in Kelvin from given Celsius temperature T = 273 + 458 = 731 K 4. Using the rela ...
... What would be the Kp of this system if R = 0.0821 liter-atm/mole K? 1. Check to make sure the equation is balanced 2. Calculate n n = Total gas moles on the right - Total gas moles on the left n=2-2=0 3. Calculate T in Kelvin from given Celsius temperature T = 273 + 458 = 731 K 4. Using the rela ...
An Analogy for an Equilibrium Reaction
... Given the following changes (assuming constant T and V): a) addition of ammonia to use up the extra ammonia the system will shift to the left b) removal of NO2 to replace some of the nitrogen dioxide the system will shift to the left c) removal of water vapour to replace some of the water vapour the ...
... Given the following changes (assuming constant T and V): a) addition of ammonia to use up the extra ammonia the system will shift to the left b) removal of NO2 to replace some of the nitrogen dioxide the system will shift to the left c) removal of water vapour to replace some of the water vapour the ...
chem 102 class notes - Louisiana Tech University
... What would be the Kp of this system if R = 0.0821 liter-atm/mole K? 1. Check to make sure the equation is balanced 2. Calculate ∆ n ∆n = Total gas moles on the right - Total gas moles on the left ∆n=2-2=0 3. Calculate T in Kelvin from given Celsius temperature T = 273 + 458 = 731 K 4. Using the rela ...
... What would be the Kp of this system if R = 0.0821 liter-atm/mole K? 1. Check to make sure the equation is balanced 2. Calculate ∆ n ∆n = Total gas moles on the right - Total gas moles on the left ∆n=2-2=0 3. Calculate T in Kelvin from given Celsius temperature T = 273 + 458 = 731 K 4. Using the rela ...
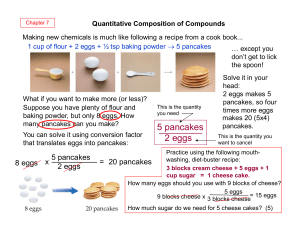
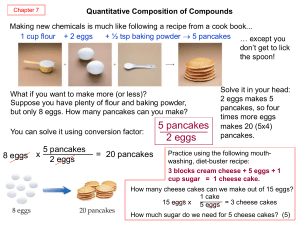
Stoichiometry
... This reaction tells us that by mixing 2 moles of sodium with 1 mole of chlorine we will get 2 moles of sodium chloride How much Na and Cl2 would be need if we wanted to make 4 moles of NaCl? ...
... This reaction tells us that by mixing 2 moles of sodium with 1 mole of chlorine we will get 2 moles of sodium chloride How much Na and Cl2 would be need if we wanted to make 4 moles of NaCl? ...
The mole concept Since Compounds are formed
... 1 mole of oxygen (atoms) is 16 g, so 0.1386 g O is 0.1386/16 = 8.663 x 10 -3 mol Dividing both amounts by the smaller number of moles yields the mole ratio of the two elements: 8.702 x 10 -3 mol / 8.663 x 10 -3 mol = 1.004 8.663 x 10 -3 mol / 8.663 x 10 -3 mol = 1.000 Within experimental error the M ...
... 1 mole of oxygen (atoms) is 16 g, so 0.1386 g O is 0.1386/16 = 8.663 x 10 -3 mol Dividing both amounts by the smaller number of moles yields the mole ratio of the two elements: 8.702 x 10 -3 mol / 8.663 x 10 -3 mol = 1.004 8.663 x 10 -3 mol / 8.663 x 10 -3 mol = 1.000 Within experimental error the M ...
Chemical Weathering on Mars
... Chemical weathering on Mars is examined theoretically from the standpoint of heterogeneous equilibrium between solid mineral phases and gaseous 02, H20, and CO2 in the Martian atmosphere. Thermochemical calculations are performed in order to identify important gas-solid decomposition reactions invol ...
... Chemical weathering on Mars is examined theoretically from the standpoint of heterogeneous equilibrium between solid mineral phases and gaseous 02, H20, and CO2 in the Martian atmosphere. Thermochemical calculations are performed in order to identify important gas-solid decomposition reactions invol ...
Absorption Spectra and Photolysis of Methyl Peroxide in Liquid and
... Kamboures et al.27 used on-the-fly ab initio dynamics on a semiempirical PM3 potential to study the photodissociation of CH3OOH adsorbed on 20 water molecules. Following the breakage of the O−O bond, secondary reactions between OH and CH3O on frozen water clusters were shown to yield formaldehyde, w ...
... Kamboures et al.27 used on-the-fly ab initio dynamics on a semiempirical PM3 potential to study the photodissociation of CH3OOH adsorbed on 20 water molecules. Following the breakage of the O−O bond, secondary reactions between OH and CH3O on frozen water clusters were shown to yield formaldehyde, w ...
A thermodynamic model for the prediction of phase equilibria and
... as a function of temperature, pressure, CO2 concentration and salinity. As we know, CO2 sequestration is considered to be a viable way to reduce the CO2 emission to the air. When injected into the underground, CO2 will have complex reactions with all the aqueous species. Accurate calculation of the ...
... as a function of temperature, pressure, CO2 concentration and salinity. As we know, CO2 sequestration is considered to be a viable way to reduce the CO2 emission to the air. When injected into the underground, CO2 will have complex reactions with all the aqueous species. Accurate calculation of the ...
The phase diagram of water at negative pressures: Virtual ices
... The screening parameter and the number of vectors of reciprocal space considered had to be carefully selected for each crystal phase.50,51 The free energies of the solid phases were evaluated by using the Einstein molecule approach proposed by Vega and Noya53 and extended to molecular systems by Noy ...
... The screening parameter and the number of vectors of reciprocal space considered had to be carefully selected for each crystal phase.50,51 The free energies of the solid phases were evaluated by using the Einstein molecule approach proposed by Vega and Noya53 and extended to molecular systems by Noy ...
Li−Fe−P−O2 Phase Diagram from First Principles Calculations
... using ab initio methods. The ground-state energies of all known compounds in the Li-Fe-P-O2 system were calculated using the generalized gradient approximation (GGA) approximation to density functional theory (DFT) and the DFT+U extension to it. Considering only the entropy of gaseous phases, the ph ...
... using ab initio methods. The ground-state energies of all known compounds in the Li-Fe-P-O2 system were calculated using the generalized gradient approximation (GGA) approximation to density functional theory (DFT) and the DFT+U extension to it. Considering only the entropy of gaseous phases, the ph ...
[HMIM][Br9]: a Room-temperature Ionic Liquid Based on a
... Crystal and molecular structure The single-crystal X-ray structure determination of [HMIM][Br9 ] (1) has shown that the salt crystallizes in the orthorhombic space group P21 21 21 with Z = 4. In analogy to other polyhalide monoanions the [Br9 ]− structure is built up of the two building blocks, Br2 ...
... Crystal and molecular structure The single-crystal X-ray structure determination of [HMIM][Br9 ] (1) has shown that the salt crystallizes in the orthorhombic space group P21 21 21 with Z = 4. In analogy to other polyhalide monoanions the [Br9 ]− structure is built up of the two building blocks, Br2 ...
1 mol H 2
... Coefficients can also represent “number of moles”! We can, therefore, interpret coefficients as numbers of moles! ...
... Coefficients can also represent “number of moles”! We can, therefore, interpret coefficients as numbers of moles! ...

















![[HMIM][Br9]: a Room-temperature Ionic Liquid Based on a](http://s1.studyres.com/store/data/016911324_1-ac5688316a1e3a6c1ba364df016e5832-300x300.png)

