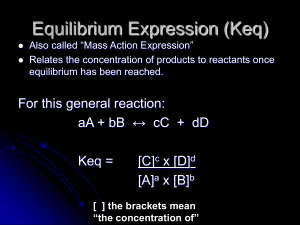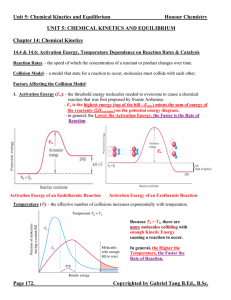
Unit 5: Chemical Kinetics and Equilibrium
... 15.2: Ways of Expressing Equilibrium Constants Homogeneous Equilibria: - an equilibrium system where all chemical species are in the same phase. Equilibrium Constant (K): - the symbol for equilibrium constant when the expression deals with concentrations is simply K or Kc. When the expression deals ...
... 15.2: Ways of Expressing Equilibrium Constants Homogeneous Equilibria: - an equilibrium system where all chemical species are in the same phase. Equilibrium Constant (K): - the symbol for equilibrium constant when the expression deals with concentrations is simply K or Kc. When the expression deals ...
Abstract - Institute of Sound and Vibration Research
... extremely important for a number of industrial and global processes.8 In bubbles this gas exchange effectively changes the size of the gas bubble and leads to growth or dissolution depending on the size of the bubble. The effects of rectified diffusion on the size of a large gas bubble can be invest ...
... extremely important for a number of industrial and global processes.8 In bubbles this gas exchange effectively changes the size of the gas bubble and leads to growth or dissolution depending on the size of the bubble. The effects of rectified diffusion on the size of a large gas bubble can be invest ...
Nucleation Rates of Supercooled Water
... • if the drop is liquid then the laser light has the same polarization as before the interaction with the drop • if the droplet freezes then the laser ...
... • if the drop is liquid then the laser light has the same polarization as before the interaction with the drop • if the droplet freezes then the laser ...
Thermodynamics and Phase Diagrams
... where kB is Boltzmann’s constant and t is the multiplicity of the system. Somewhat loosely, t is the number of possible equivalent microstates in a macrostate, that is the number of quantum states for the system which are accessible under the applicable conditions of energy, volume, etc. For example ...
... where kB is Boltzmann’s constant and t is the multiplicity of the system. Somewhat loosely, t is the number of possible equivalent microstates in a macrostate, that is the number of quantum states for the system which are accessible under the applicable conditions of energy, volume, etc. For example ...
JCA 2007 (vol 1159, pp 51-57)
... parameters like sheath liquid flow rate, nebulizing gas pressure, capillary sprayer position and drying gas flow and temperature, were studied. In order to mimic the conditions during NAEKC-MS analyses, this was done by voltage-induced infusion of the test compound mebev-erine in the BGE. A BGE of 1 ...
... parameters like sheath liquid flow rate, nebulizing gas pressure, capillary sprayer position and drying gas flow and temperature, were studied. In order to mimic the conditions during NAEKC-MS analyses, this was done by voltage-induced infusion of the test compound mebev-erine in the BGE. A BGE of 1 ...
Nafion® Dryers and Humidifiers The Ten Most Common Questions
... The secondary effect of temperature on Nafion function relates to the final equilibrium point. For drying or humidification to occur, there must be a water vapor pressure gradient across the tubing wall. Drying/humidification stops when there is no longer a gradient; at this point equilibrium has b ...
... The secondary effect of temperature on Nafion function relates to the final equilibrium point. For drying or humidification to occur, there must be a water vapor pressure gradient across the tubing wall. Drying/humidification stops when there is no longer a gradient; at this point equilibrium has b ...
Answer
... • In the spaces provided, explain the meanings of the following terms. You may use an equation or diagram where appropriate. (a) hydrogen bonding An unusually strong dipole-dipole interaction that forms when a hydrogen atom is bonded to one of the very electronegative atoms F, O or N. ...
... • In the spaces provided, explain the meanings of the following terms. You may use an equation or diagram where appropriate. (a) hydrogen bonding An unusually strong dipole-dipole interaction that forms when a hydrogen atom is bonded to one of the very electronegative atoms F, O or N. ...
File
... Hydrates are ionic compounds (salts) that have a definite amount of water as part of their structure. This “water of hydration” is released as vapor when the hydrate is heated. The remaining salt is known as the anhydrous salt. The general reaction for heating a hydrate is: CoCl2·6H2O + heat ...
... Hydrates are ionic compounds (salts) that have a definite amount of water as part of their structure. This “water of hydration” is released as vapor when the hydrate is heated. The remaining salt is known as the anhydrous salt. The general reaction for heating a hydrate is: CoCl2·6H2O + heat ...
Thermophysical Properties of High-Temperature Reacting Mixtures
... Abstract This paper is devoted to the calculation of the chemical equilibrium composition and thermodynamic properties of reacting mixtures of carbon and water at high temperature. Equilibrium particle concentrations and thermodynamic properties including mass density, molar weight, entropy, enthalp ...
... Abstract This paper is devoted to the calculation of the chemical equilibrium composition and thermodynamic properties of reacting mixtures of carbon and water at high temperature. Equilibrium particle concentrations and thermodynamic properties including mass density, molar weight, entropy, enthalp ...
2008 Equilibrium -- without math (PowerPoint 13 MB)
... between forward and reverse reactions. In most cases, this balance is quite delicate. Changes in experimental conditions (concentration, pressure, volume and temperature) may disturb the balance and shift the equilibrium position so that more or less of the desired product is formed. When we say tha ...
... between forward and reverse reactions. In most cases, this balance is quite delicate. Changes in experimental conditions (concentration, pressure, volume and temperature) may disturb the balance and shift the equilibrium position so that more or less of the desired product is formed. When we say tha ...
Reactions and Solutions - Louisiana Tech University
... solutions, the solvent is water. Liquid solutions are clear and transparent with no visible particles of solute. They may be colored or colorless, depending on the properties of the solute and solvent. In solutions of electrolytes, the solutes are ionic compounds that have dissociated; the solution ...
... solutions, the solvent is water. Liquid solutions are clear and transparent with no visible particles of solute. They may be colored or colorless, depending on the properties of the solute and solvent. In solutions of electrolytes, the solutes are ionic compounds that have dissociated; the solution ...
Auto-ignition Characteristics of Selected Ionic Liquids
... according to their flash point, the flammability hazard of a material should not be defined by a single flammability test [5]. Heat release characteristics of ionic liquids are investigated in their work and they concluded that it would be more appropriate to describe ionic liquids as having low or ...
... according to their flash point, the flammability hazard of a material should not be defined by a single flammability test [5]. Heat release characteristics of ionic liquids are investigated in their work and they concluded that it would be more appropriate to describe ionic liquids as having low or ...
Liquid Mixtures Involving Hydrogenated and Fluorinated Alcohols
... induce organization. We have accomplished a systematic study of perfluoroalkylalkanes (PFAA).3−6 These diblock compounds, made of alkyl and perfluoroalkyl segments covalently bonded to form a single chain, display amphiphilic character7,8 and the orientational ordering of smectogenic liquid crystals.9 ...
... induce organization. We have accomplished a systematic study of perfluoroalkylalkanes (PFAA).3−6 These diblock compounds, made of alkyl and perfluoroalkyl segments covalently bonded to form a single chain, display amphiphilic character7,8 and the orientational ordering of smectogenic liquid crystals.9 ...
Unit 3 2 Basic Mole Conversions and Mole Maps
... Meaning: For every 2 mol of ethane, 7 mol of dioxygen molecules are consumed in the combustion (a ratio of 2 to 7) This produces 4 moles of carbon dioxide, 6 moles of water and releases 3,170 kJ of energy from the bonding chemicals to the environment. This set of relationships can indicate, at a gla ...
... Meaning: For every 2 mol of ethane, 7 mol of dioxygen molecules are consumed in the combustion (a ratio of 2 to 7) This produces 4 moles of carbon dioxide, 6 moles of water and releases 3,170 kJ of energy from the bonding chemicals to the environment. This set of relationships can indicate, at a gla ...
Lecture 8 Laminar Diffusion Flames: Diffusion Flamelet Theory
... To leading order one obtains the adiabatic flame temperature which is a function of mixture fraction only. The asymptotic expansion around this limit will then describe the influence of finite rate chemistry. If the expansion takes the temperature sensitivity of the chemistry into account diffusion ...
... To leading order one obtains the adiabatic flame temperature which is a function of mixture fraction only. The asymptotic expansion around this limit will then describe the influence of finite rate chemistry. If the expansion takes the temperature sensitivity of the chemistry into account diffusion ...
Predicting Moles of Reactants/Products
... Goal: To make 0.5 g of CuO. (And to make an educated guess as to the amount of starting material that will give us 0.5 g of CuO.) Safety: Tie back long hair and loose clothing near the Bunsen burner. Copper carbonate is toxic. Do not ingest it and wear goggles at all times. Be careful with the flame ...
... Goal: To make 0.5 g of CuO. (And to make an educated guess as to the amount of starting material that will give us 0.5 g of CuO.) Safety: Tie back long hair and loose clothing near the Bunsen burner. Copper carbonate is toxic. Do not ingest it and wear goggles at all times. Be careful with the flame ...
Fall 2006
... moles, and temperature of the gas are noted on the diagram. Which diagram (2)-(4) most closely represents the position of the piston when the pressure and the number of moles of gas are both doubled while keeping the temperature constant? ...
... moles, and temperature of the gas are noted on the diagram. Which diagram (2)-(4) most closely represents the position of the piston when the pressure and the number of moles of gas are both doubled while keeping the temperature constant? ...
Ch 6 LAN 7th Intro Chem Chemical Reactions
... The combustion of acetylene gas (C2H2) produces carbon dioxide and water as indicated in the following reaction 2 C2H2 (g) + 5 O2 (g) → 4 CO2 (g) + 2 H2O (g) When 26.0 g of acetylene is burned in sufficient oxygen for complete reaction, the theoretical yield of CO2 is 88.0 g Calculate the percent yi ...
... The combustion of acetylene gas (C2H2) produces carbon dioxide and water as indicated in the following reaction 2 C2H2 (g) + 5 O2 (g) → 4 CO2 (g) + 2 H2O (g) When 26.0 g of acetylene is burned in sufficient oxygen for complete reaction, the theoretical yield of CO2 is 88.0 g Calculate the percent yi ...
DEPARTMENT OF CHEMISTRY AND CHEMICAL TECHNOLOGY
... Print your name: _____________________________________________ ...
... Print your name: _____________________________________________ ...