3.2 Notes
... create an equation that is equivalent to the original equation. Equivalent equations have the same solutions, or the same solution set. In the example above, 2x + 5 = 11, 2x = 6, and x = 3 are all equivalent equations. ...
... create an equation that is equivalent to the original equation. Equivalent equations have the same solutions, or the same solution set. In the example above, 2x + 5 = 11, 2x = 6, and x = 3 are all equivalent equations. ...
The first law of thermodynamics
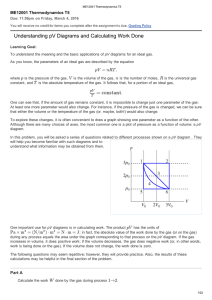
... Deduce an expression for the work involved in a volume change of a gas at constant pressure. State the first law of thermodynamics. Identify the first law of thermodynamics as a statement of the principle of energy conservation. Describe the isochoric (isovolumetric), isobaric, isothermal an ...
... Deduce an expression for the work involved in a volume change of a gas at constant pressure. State the first law of thermodynamics. Identify the first law of thermodynamics as a statement of the principle of energy conservation. Describe the isochoric (isovolumetric), isobaric, isothermal an ...
Conjugated Polymer Superlattices for Enhanced
... prohibitive cost of mass production. Organic materials offer an exciting economical alternative because of their flexibility and low manufacturing cost. Since organic photovoltaics (OPVs) are much cheaper to fabricate, improving OPV efficiency might provide an economical and sustainable solution for ...
... prohibitive cost of mass production. Organic materials offer an exciting economical alternative because of their flexibility and low manufacturing cost. Since organic photovoltaics (OPVs) are much cheaper to fabricate, improving OPV efficiency might provide an economical and sustainable solution for ...
Real gas CFD simulations of hydrogen/oxygen supercritical
... the mean free path between the molecules in the §uid becomes small enough to allow molecular interactions to become important. This leads to signi¦cant deviations from the ideal gas assumption, which entirely neglects intermolecular attraction and repulsion e¨ects. For this reason, proper real gas r ...
... the mean free path between the molecules in the §uid becomes small enough to allow molecular interactions to become important. This leads to signi¦cant deviations from the ideal gas assumption, which entirely neglects intermolecular attraction and repulsion e¨ects. For this reason, proper real gas r ...