ATOMIC MASS
... Matter (space & mass) Element; compound The atom Atomic number (# of protons); mass number (protons + neutrons) ...
... Matter (space & mass) Element; compound The atom Atomic number (# of protons); mass number (protons + neutrons) ...
The Modern Theory of Atomic Structure
... Isotopes are: Different forms of the same element with the SAME # of Protons but with DIFFERENT #’s of Neutrons ...
... Isotopes are: Different forms of the same element with the SAME # of Protons but with DIFFERENT #’s of Neutrons ...
Elements and Atoms
... The reason different elements have different properties is because the atoms that make up different elements have different numbers of tiny particles that make them up. The particles that make up atoms are called subatomic particles. There are three types of subatomic particles: 1. protons (positive ...
... The reason different elements have different properties is because the atoms that make up different elements have different numbers of tiny particles that make them up. The particles that make up atoms are called subatomic particles. There are three types of subatomic particles: 1. protons (positive ...
ATOMS / ATOMIC STRUCTURE NOTES Atom
... Atomic number-the number of protons in the nucleus of an atom, it determines the identity of the element Atomic weight-the average number of protons plus neutrons in the nucleus of an atom Atomic mass unit(AMU)-the average weight of one proton or neutron Isotope-an atom of the same element but with ...
... Atomic number-the number of protons in the nucleus of an atom, it determines the identity of the element Atomic weight-the average number of protons plus neutrons in the nucleus of an atom Atomic mass unit(AMU)-the average weight of one proton or neutron Isotope-an atom of the same element but with ...
Answers
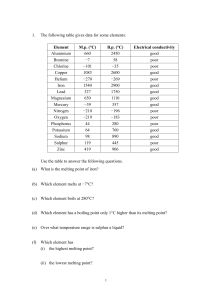
... (b) All are soft metals. [1] (c) Lithium would float on water, [1] producing gas steadily. [1] (d) Potassium would melt to a silvery ball [1] which moves about very quickly on the water surface, [1] producing a hissing sound, [1] burning spontaneously with a lilac flame [1] before finally disappeari ...
... (b) All are soft metals. [1] (c) Lithium would float on water, [1] producing gas steadily. [1] (d) Potassium would melt to a silvery ball [1] which moves about very quickly on the water surface, [1] producing a hissing sound, [1] burning spontaneously with a lilac flame [1] before finally disappeari ...
Alpha Beta Fission Fusion
... a T½ of five days. Bi215, by comparison, undergoes decay to Po215 with a T½ of 7.6 minutes, and Bi208 undergoes yet another mode of radioactive decay (called electron capture) with a T½ of 368,000 years! Stimulated Nuclear Reactions While many elements undergo radioactive decay naturally, nuclear ...
... a T½ of five days. Bi215, by comparison, undergoes decay to Po215 with a T½ of 7.6 minutes, and Bi208 undergoes yet another mode of radioactive decay (called electron capture) with a T½ of 368,000 years! Stimulated Nuclear Reactions While many elements undergo radioactive decay naturally, nuclear ...
Atomic Structure and Types of Atoms
... is the sum of the protons and neutrons in the nucleus of an atom. The most common isotope of carbon has a mass number of 12 (6 protons + 6 neutrons) and may be written as “carbon-12.” Two other isotopes are carbon-13 and carbon-14. As shown in Figure 3, a symbol with the mass number above and the at ...
... is the sum of the protons and neutrons in the nucleus of an atom. The most common isotope of carbon has a mass number of 12 (6 protons + 6 neutrons) and may be written as “carbon-12.” Two other isotopes are carbon-13 and carbon-14. As shown in Figure 3, a symbol with the mass number above and the at ...
Atomic Structure Notes
... □ Horizontal Rows (label these from top to bottom #’s 1 through 7. The row number ...
... □ Horizontal Rows (label these from top to bottom #’s 1 through 7. The row number ...
Picture Match Words Fusion Density Isotope Neutron Atomic
... d. Grade own understanding (using the Vocab Journal on a scale of 1-4) Answer Key ...
... d. Grade own understanding (using the Vocab Journal on a scale of 1-4) Answer Key ...
Summative Assessment Review!
... • An alpha particle is simply a helium nuclei (He) which is ejected with high energy from an unstable nucleus ...
... • An alpha particle is simply a helium nuclei (He) which is ejected with high energy from an unstable nucleus ...
UNIT 2 – THE ATOM - Neshaminy School District
... All the mass of the atom is in the nucleus since that is where the protons and neutrons are and they are the only particles that have mass. You can determine the atom’s mass using the following equation. ...
... All the mass of the atom is in the nucleus since that is where the protons and neutrons are and they are the only particles that have mass. You can determine the atom’s mass using the following equation. ...
OCR A Level Physics B Delivery Guide Learner Resource 1: Atomic
... The number x is often called the mass number and gives an indication of the relative mass of the atom. It can also be called the nucleon number because it is equal to the number of neutrons and protons in the nucleus of the atom. The word nucleon covers both neutrons and protons. The number z is the ...
... The number x is often called the mass number and gives an indication of the relative mass of the atom. It can also be called the nucleon number because it is equal to the number of neutrons and protons in the nucleus of the atom. The word nucleon covers both neutrons and protons. The number z is the ...
Atomic Structure
... number of protons in the nucleus Also the number of electrons in a neutral atom Located just above the element symbol on the PT (whole number) Elements are listed on the PT according to their atomic number increasing from left to right ...
... number of protons in the nucleus Also the number of electrons in a neutral atom Located just above the element symbol on the PT (whole number) Elements are listed on the PT according to their atomic number increasing from left to right ...
Thomson`s Atom
... Atomic weights do not always increase with atomic number, i.e. position in Periodic table: Atomic weight of Argon (Ar) exceeds that of the succeeding element Potassium (K) Atomic weight of Cobalt (Co) exceeds that of the succeeding element Nickel (Ni) Pb from different localities has different atomi ...
... Atomic weights do not always increase with atomic number, i.e. position in Periodic table: Atomic weight of Argon (Ar) exceeds that of the succeeding element Potassium (K) Atomic weight of Cobalt (Co) exceeds that of the succeeding element Nickel (Ni) Pb from different localities has different atomi ...
CHAPTER 4: ATOMS AND ELEMENTS
... 4.1 Experiencing Atoms at Tiburon atom: smallest identifiable unit of an element – All matter is made up of atoms. → The properties of specific atoms determine the properties of matter with those atoms. There are currently 91 naturally occurring elements and 20 man-made elements. 4.2 Indivisible: Th ...
... 4.1 Experiencing Atoms at Tiburon atom: smallest identifiable unit of an element – All matter is made up of atoms. → The properties of specific atoms determine the properties of matter with those atoms. There are currently 91 naturally occurring elements and 20 man-made elements. 4.2 Indivisible: Th ...
File
... The normal atom (also called a stable atom) has three main parts (or particles) that we are concerned with; protons, neutrons, and electrons. A fourth part, the nucleus, is often referred to but we are not focusing on it as much as we are on protons, neutrons, and electrons. The nucleus is the centr ...
... The normal atom (also called a stable atom) has three main parts (or particles) that we are concerned with; protons, neutrons, and electrons. A fourth part, the nucleus, is often referred to but we are not focusing on it as much as we are on protons, neutrons, and electrons. The nucleus is the centr ...
Isotope

Isotopes are variants of a particular chemical element which differ in neutron number, although all isotopes of a given element have the same number of protons in each atom. The term isotope is formed from the Greek roots isos (ἴσος ""equal"") and topos (τόπος ""place""), meaning ""the same place""; thus, the meaning behind the name it is that different isotopes of a single element occupy the same position on the periodic table. The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively.