GEO143_activity_2
... Number of Protons = Atomic Number (Use the large colored marshmallows for protons) Number of Neutrons = Atomic Mass – Atomic Number (Use the large white marshmallows for neutrons) Number of Electrons = Number of Protons (Use the small colored marshmallows for electrons) ...
... Number of Protons = Atomic Number (Use the large colored marshmallows for protons) Number of Neutrons = Atomic Mass – Atomic Number (Use the large white marshmallows for neutrons) Number of Electrons = Number of Protons (Use the small colored marshmallows for electrons) ...
Properties of Atoms and the Periodic Table
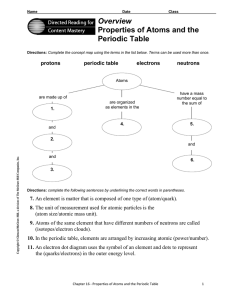
... Directions: Complete the concept map using the terms in the list below. Terms can be used more than once, protons ...
... Directions: Complete the concept map using the terms in the list below. Terms can be used more than once, protons ...
Education TI - Texas Instruments
... nucleus comprised of protons and neutrons surrounded by electrons. In this model, electrons orbit the nucleus in circular paths at different distances called electron shells. This model became popular because it fit the experimental results for Hydrogen. Later, the application of the model to heavie ...
... nucleus comprised of protons and neutrons surrounded by electrons. In this model, electrons orbit the nucleus in circular paths at different distances called electron shells. This model became popular because it fit the experimental results for Hydrogen. Later, the application of the model to heavie ...
Summary of lesson
... nucleus comprised of protons and neutrons surrounded by electrons. In this model, electrons orbit the nucleus in circular paths at different distances called electron shells. This model became popular because it fit the experimental results for Hydrogen. Later, the application of the model to heavie ...
... nucleus comprised of protons and neutrons surrounded by electrons. In this model, electrons orbit the nucleus in circular paths at different distances called electron shells. This model became popular because it fit the experimental results for Hydrogen. Later, the application of the model to heavie ...
Unit 4 Packet
... 12. How do the three isotopes of hydrogen (H–1, H–2, H–3) compare in terms of the numbers of subatomic particles in each? 13. Write the nuclear symbol for deuterium (H-2): a. Identify the atomic number b. Identify the mass number 14. Determine the number of protons, neutrons, and electrons in Co–59. ...
... 12. How do the three isotopes of hydrogen (H–1, H–2, H–3) compare in terms of the numbers of subatomic particles in each? 13. Write the nuclear symbol for deuterium (H-2): a. Identify the atomic number b. Identify the mass number 14. Determine the number of protons, neutrons, and electrons in Co–59. ...
Atom notes - WordPress.com
... 2. The neutron was basically equal in mass to the proton but had _______ ____________________ charge. NEILS BOHR (1914) 1. Concluded that ________________ moved around the nucleus in definite orbits or ___________________________. DALTON REVISITED 1. Atom was indivisible. _____________ 2. All elemen ...
... 2. The neutron was basically equal in mass to the proton but had _______ ____________________ charge. NEILS BOHR (1914) 1. Concluded that ________________ moved around the nucleus in definite orbits or ___________________________. DALTON REVISITED 1. Atom was indivisible. _____________ 2. All elemen ...
ATOM ATOMIC SYMBOL ATOMIC NUMBER
... (2) 5 pts ‐ Refer to a Periodic Table and the Key below to fill out this table for each element. Turn Lithium atom into an ion and note the information. Turn Beryllium into an isotope and record what you did. ...
... (2) 5 pts ‐ Refer to a Periodic Table and the Key below to fill out this table for each element. Turn Lithium atom into an ion and note the information. Turn Beryllium into an isotope and record what you did. ...
e - Humble ISD
... b) Neutrons are neutral and defines the isotopes. c) Electrons are –ve and defines the chemical properties of the element. 4. Isotopes are atoms of the same element that differ in the number of neutrons. 5. The Atomic Number of an atom = number of protons in the nucleus. 6. The Atomic Mass of an ato ...
... b) Neutrons are neutral and defines the isotopes. c) Electrons are –ve and defines the chemical properties of the element. 4. Isotopes are atoms of the same element that differ in the number of neutrons. 5. The Atomic Number of an atom = number of protons in the nucleus. 6. The Atomic Mass of an ato ...
Lap 4: Atomic Structure Mead Chemistry Chapter 4 4.1 Defining the
... c. If plum pudding theory is true, particles should pass easily through with only slight deflection when positive particles hit the spread out positive protons 3. Did not happen as expected a. Instead most particles passed easily through b. Small number bounced back at very large angle 4. Rutherford ...
... c. If plum pudding theory is true, particles should pass easily through with only slight deflection when positive particles hit the spread out positive protons 3. Did not happen as expected a. Instead most particles passed easily through b. Small number bounced back at very large angle 4. Rutherford ...
Atomic Mass - Coach ONeal
... • The mass of a proton is about the same as that of a neutron. And the mass of each is about 1,800 times greater than the mass of the electron. • The unit of measurement used for atomic particles is the atomic mass unit (amu). • The mass of a proton or a neutron is almost equal to 1 amu. ...
... • The mass of a proton is about the same as that of a neutron. And the mass of each is about 1,800 times greater than the mass of the electron. • The unit of measurement used for atomic particles is the atomic mass unit (amu). • The mass of a proton or a neutron is almost equal to 1 amu. ...
Experiment Name - suzhoualevelphysics
... • State the meaning of radioactive decay, using equations (involving words or symbols) to represent changes in the composition of the nucleus when particles are emitted ...
... • State the meaning of radioactive decay, using equations (involving words or symbols) to represent changes in the composition of the nucleus when particles are emitted ...
Origin of the Atom
... 3) An atom is held together by electric forces. This is from the attractive force of the electrons(-) and the ...
... 3) An atom is held together by electric forces. This is from the attractive force of the electrons(-) and the ...
CHAPTER 13: Nuclear Interactions and Applications
... Fission neutrons typically have 1–2 MeV of kinetic energy, and because the fission cross section increases as 1/v at low energies, slowing down the neutrons helps to increase the chance of producing another fission. A moderator is used to elastically scatter the high-energy neutrons and thus reduce ...
... Fission neutrons typically have 1–2 MeV of kinetic energy, and because the fission cross section increases as 1/v at low energies, slowing down the neutrons helps to increase the chance of producing another fission. A moderator is used to elastically scatter the high-energy neutrons and thus reduce ...
Atomic Structure
... 22. If the atomic weight of an element is 23 times that of the lightest element and it has 11 protons, then it contains: a. 11 protons, 23 neutrons, 11 electrons b. 11 protons, 12 neutrons, 11 electrons c. 11 protons, 11 neutrons, 11 electrons d. 11 protons, 11 neutrons, 23 electrons. 23. According ...
... 22. If the atomic weight of an element is 23 times that of the lightest element and it has 11 protons, then it contains: a. 11 protons, 23 neutrons, 11 electrons b. 11 protons, 12 neutrons, 11 electrons c. 11 protons, 11 neutrons, 11 electrons d. 11 protons, 11 neutrons, 23 electrons. 23. According ...
The Structure of an Atom
... This number identifies the element Periodic table is arranged in sequence of increasing atomic numbers Atoms are neutral: (+) charges must equal (-) charges ...
... This number identifies the element Periodic table is arranged in sequence of increasing atomic numbers Atoms are neutral: (+) charges must equal (-) charges ...
Energy Basics
... the nuclei in a given quantity of a radioisotope to decay and emit their radiation to form a different isotope. Decay continues, often producing a series of different radioisotopes, until a stable, nonradioactive isotope is formed. The half-life estimates how long a sample of radioactive isotope ...
... the nuclei in a given quantity of a radioisotope to decay and emit their radiation to form a different isotope. Decay continues, often producing a series of different radioisotopes, until a stable, nonradioactive isotope is formed. The half-life estimates how long a sample of radioactive isotope ...
atomic number
... Neutral atoms have the same number of protons and electrons. Ions are charged atoms. -cations – have more protons than electrons and are positively charged -anions – have more electrons than protons and are negatively charged ...
... Neutral atoms have the same number of protons and electrons. Ions are charged atoms. -cations – have more protons than electrons and are positively charged -anions – have more electrons than protons and are negatively charged ...
Average Atomic Mass
... 1. All matter is composed of extremely small particles called atoms. (atom: the smallest particle of an element that retains the chemical and physical properties of that element.) 2. Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in siz ...
... 1. All matter is composed of extremely small particles called atoms. (atom: the smallest particle of an element that retains the chemical and physical properties of that element.) 2. Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in siz ...
Overview Properties of Atoms and the Periodic Table
... 8. The unit of measurement used for atomic particles is the (atom size/atomic mass unit). 9. Atoms of the same element that have different numbers of neutrons are called (isotopes/electron clouds). 10. In the periodic table, elements are arranged by increasing atomic (power/number). 11. An electron ...
... 8. The unit of measurement used for atomic particles is the (atom size/atomic mass unit). 9. Atoms of the same element that have different numbers of neutrons are called (isotopes/electron clouds). 10. In the periodic table, elements are arranged by increasing atomic (power/number). 11. An electron ...